Acceptance of and Adherence to a Four-Dose RTS,S/AS01 Schedule: Findings from a Longitudinal Qualitative Evaluation Study for the Malaria Vaccine Implementation Programme
Abstract
:1. Introduction
2. Study Design and Methods
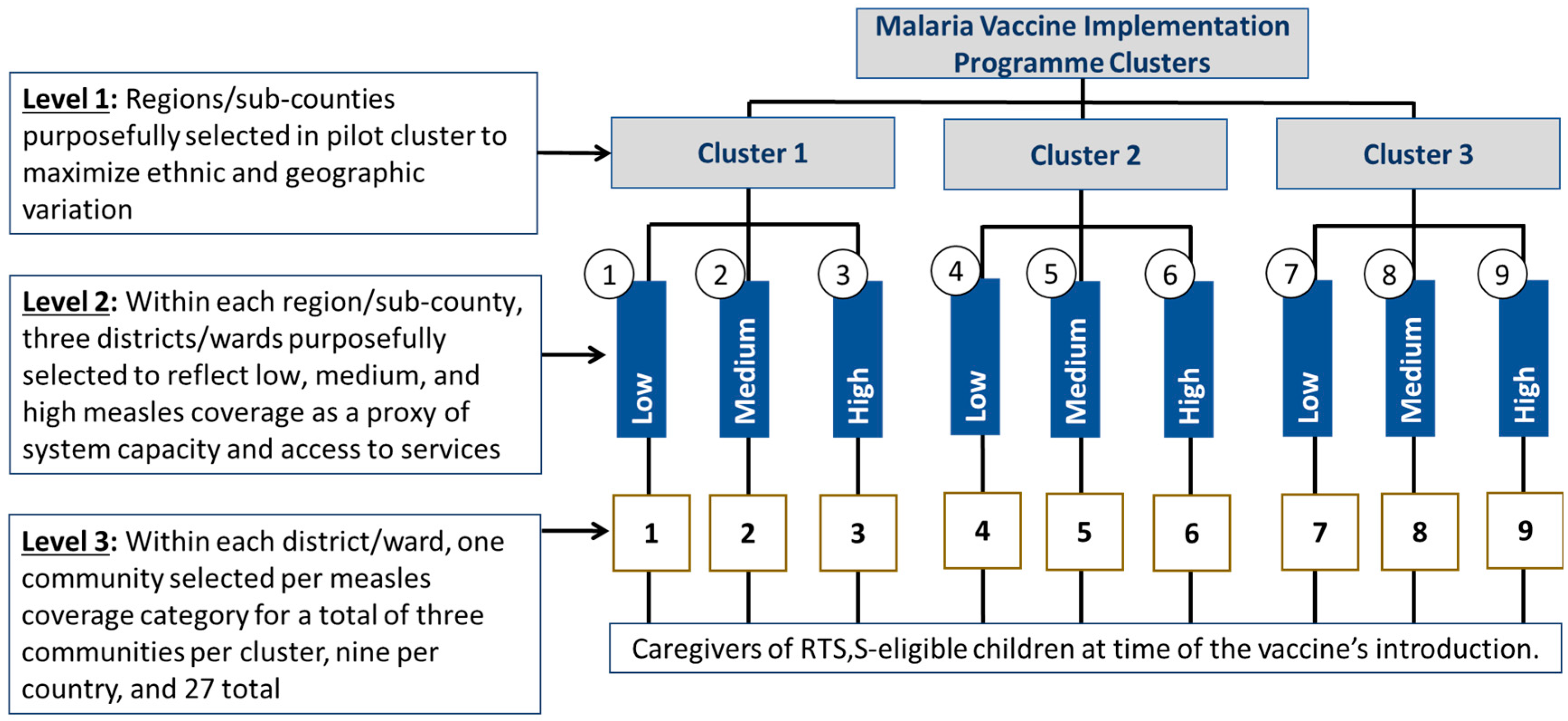
2.1. Study Sites
2.2. Child Caregiver Cohort
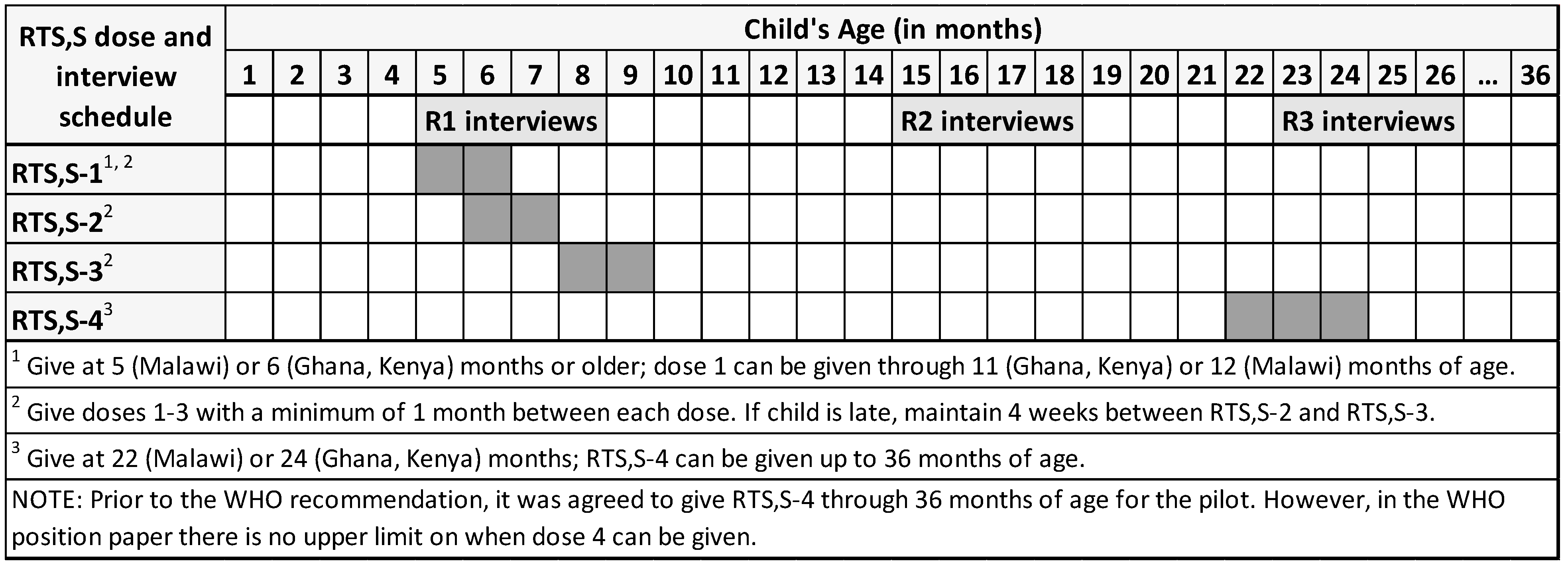
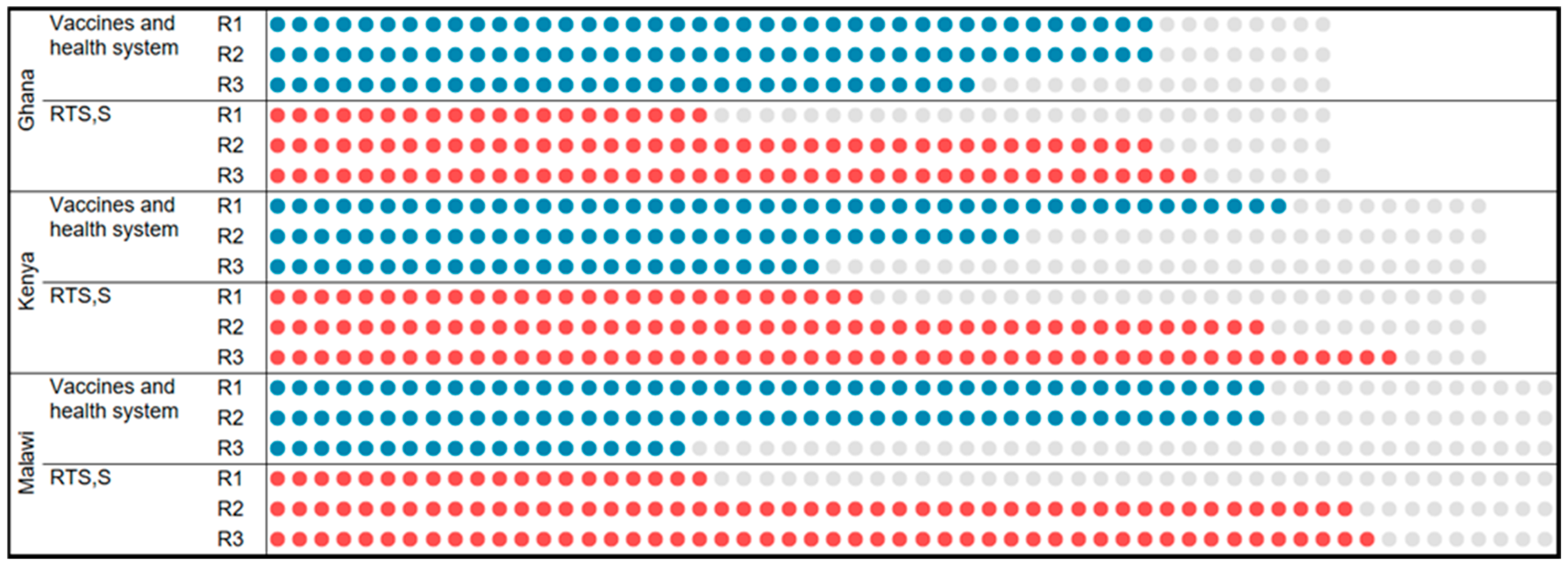
2.3. Data Collection
2.4. Data Processing and Analysis
3. Findings
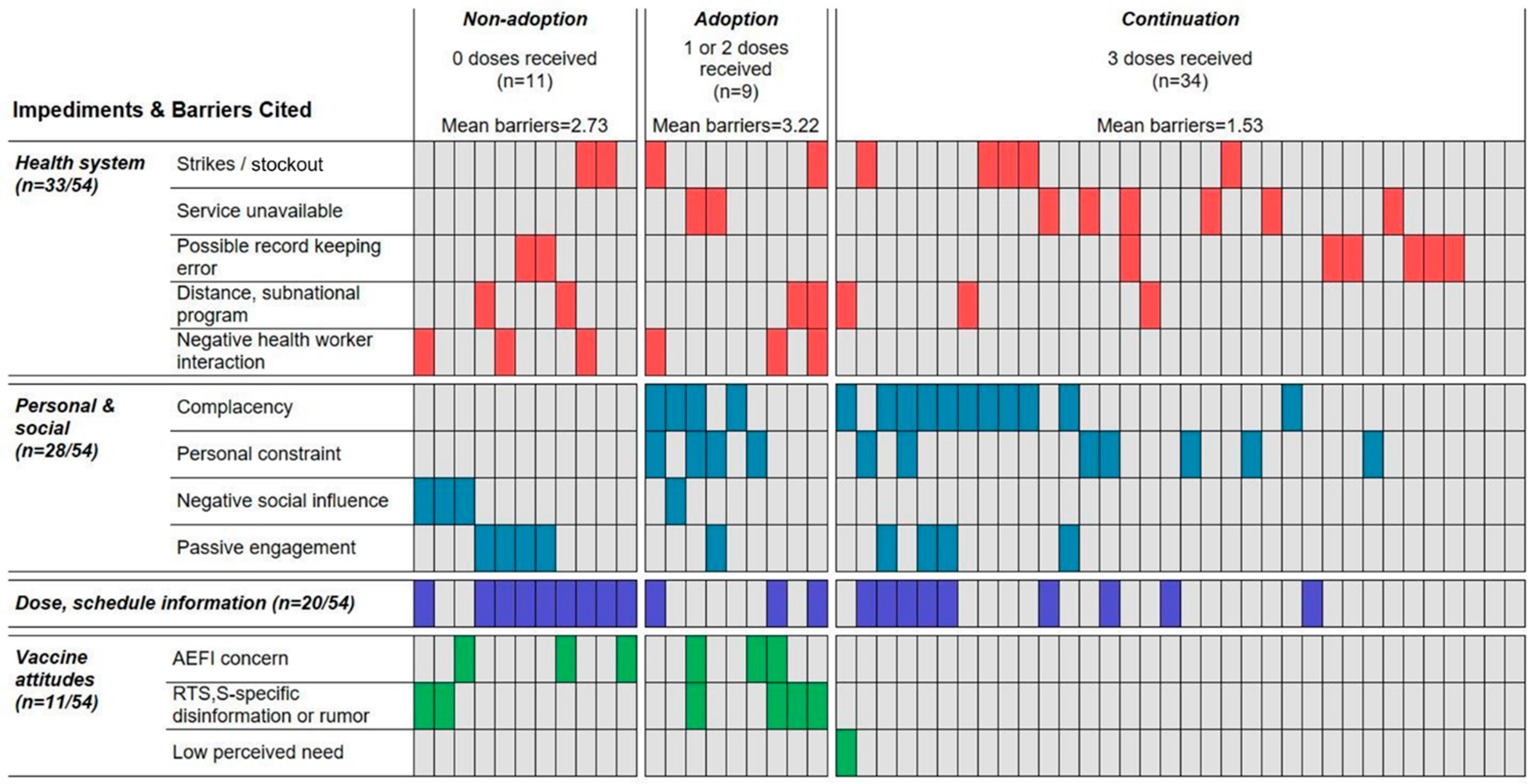
3.1. RTS,S Uptake
3.2. Uptake Facilitators
3.2.1. A Trajectory of Trust
“Why [is] something that will help children being given in only three regions”? (G_C5_002_R1)
“The concern I first had was because people were saying this new malaria vaccine is bad, that they were researching it… I got to a point when I let this concern go, because I know when hospitals come with an intervention, they’ve tried it out and start giving it after it’s certified to be good”. (M_C21_021_R1)
“They said there’s going to be a new malaria vaccine and we mothers shouldn’t panic… The nurses have gone to school and know what they’re talking about. So we became calm and accepted it”. (G_C6_002_R1)
“When I go to the hospital, I only see adults now, not children”. (M_C25_047_R3, child received 0 doses)
“If I sit down and analyze things, I know that malaria in my child is not severe like it used to be”. (G_C4_003_R3, child received 4 RTS,S doses)
3.2.2. Four-Dose Cases: Reasons for Completing the Schedule
“When they told us about the vaccine we didn’t understand. This made my mother-in-law forbid us from taking the child, fearing [RTS,S] might kill her. Then the child’s father told me to take her since it’s the nurses who brought the vaccine”. (G_C1_002_R1, child received all four doses)
“She used to get sick almost every month. After the first dose it started reducing slowly to the second then the third and fourth. From January she has not been sick”. (K_C13_006_R3)
3.3. Barriers to Adoption, Continuation, or Completion
3.3.1. Three-Dose Cases: Reasons for Non-Completion
“I told the doctor my child hadn’t received his last dose, so they jabbed him and forgot to write it down. There were a lot of people and they were working fast because of Corona. After the jab they said he finished the doses”. (M_C27_062_R3)
“I was expecting she’d be vaccinated at the under-five clinic, so I thought it was finished… I did not ask”. (M_C23_030_R3)
“I know she was supposed to take four doses, but I travelled. If not for that I would have taken her. However, I think 3 doses are protecting her because she’s never been sick with malaria since she started the vaccine”. (G_C6_005_R3)
“When I was still giving birth, I just knew children only get one dose… Now they tell me there are four, others say three. I’m not sure what’s true… I thought at three he had completed. When [child’s] mother was here I told her to take him because he’s heavy for me. She went but found the clinic closed [strike] and was told to go to a private facility. She went there and was told there was no malaria vaccine. Then [child’s mother] traveled… I got tired and don’t even know where that private hospital is”. (K_C15_003_R, child’s grandmother)
“He always receives [vaccination] in good time. Only one [RTS,S dose] is remaining but when I went, I was told to come next month. I’ve been busy with planting season, so I left that on hold”. (M_C24_040_R3)
“As parents we don’t count how many doses she received today, or anything. No, once we give birth, we leave the child in the hands of health providers. Anything to do with their health is to be done by them”. (M_C23_029_R3)
3.3.2. One-/Two-Dose Cases: Reasons for Non-Continuation
3.3.3. Zero-RTS,S-Dose Cases: Reasons for Non-Adoption
“I know how malaria cases used to be compared to now… Because of how cases are reducing, I thought I made a mistake. To be sincere, malaria has reduced”. (G_C2_007_R3)
“The nurse told me, and I saw him give two [injections] on one leg and one on the other”. (G_C2_006_R3).
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030, 2021 Update; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- RTS, S Clinical Trials Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- World Health Organization. Malaria vaccine: WHO position paper—March 2022. Wkly. Epidemiol. Rec. 2022, 97, 61–80. [Google Scholar]
- World Health Organization. Background Paper: Full Evidence Report on the RTS, S/AS01 Malaria Vaccine. Available online: https://cdn.who.int/media/docs/default-source/immunization/mvip/full-evidence-report-on-the-rtss-as01-malaria-vaccine-for-sage-mpag-(sept2021).pdf?sfvrsn=c9737be_5 (accessed on 16 November 2023).
- Abbas, K.M.; Procter, S.R.; Zandvoort, K.v.; Clark, A.; Funk, S.; Mengistu, T.; Hogan, D.; Dansereau, E.; Jit, M.; Jit, M.; et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: A benefit–risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob. Health 2020, 8, e1264–e1272. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Abbas, K.; Huber, J.; Karachaliou, A.; Thakkar, N.; Woodruff, K.; Li, X.; Echeverria-Londono, S.; Ferrari, M.; Jackson, M.L.; et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. eLife 2021, 10, e67023. [Google Scholar] [CrossRef] [PubMed]
- Sandelowski, M. Whatever happened to qualitative description? Res. Nurs. Health 2000, 23, 334–340. [Google Scholar] [CrossRef]
- Sandelowski, M. What’s in a name? Qualitative description revisited. Res. Nurs. Health 2010, 33, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.; Atkinson, S.; Doody, O. Employing a Qualitative Description Approach in Health Care Research. Glob. Qual. Nurs. Res. 2017, 4, 2333393617742282. [Google Scholar] [CrossRef] [PubMed]
- Herriott, R.E.; Firestone, W.A. Multisite Qualitative Policy Research: Optimizing Description and Generalizability. Educ. Res. 1983, 12, 14–19. [Google Scholar] [CrossRef]
- Hunt, G.; Moloney, M.; Fazio, A. Embarking on large-scale qualitative research: Reaping the benefits of mixed methods in studying youth, clubs and drugs. Nord. Stud. Alcohol Drugs 2011, 28, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Onwuegbuzie, A.J.; Leech, N.L. Sampling Designs in Qualitative Research: Making the Sampling Process More Public. Qual. Rep. 2007, 12, 238–254. [Google Scholar] [CrossRef]
- Saldaña, J. Longitudinal Qualitative Research: Analyzing Change through Time; Altamira Press: Walnut Creek, CA, USA, 2003. [Google Scholar]
- Neale, B. What is Qualitative Longitudinal Research? 1st ed.; Bloomsbury Academic: London, UK, 2016; p. 164. [Google Scholar] [CrossRef]
- Asante, K.P.; Mathanga, D.P.; Milligan, P.; Akech, S.; Oduro, A.; Mwapasa, V.; Moore, K.A.; Kwambai, T.K.; Hamel, M.J.; Gyan, T.; et al. Feasibility, Safety, and Impact of the RTS, S/AS01E Malaria Vaccine when Implemented through National Immunisation Programmes: Evaluation of Cluster-Randomized Introduction of the Vaccine in Ghana, Kenya and Malawi. Lancet 2023, in press.
- QSR International Pty Ltd. NVivo (Released in March 2020). Available online: https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home (accessed on 22 November 2023).
- Benzer, J.K.; Beehler, S.; Cramer, I.E.; Mohr, D.C.; Charns, M.P.; Burgess, J.F., Jr. Between and within-site variation in qualitative implementation research. Implement Sci. 2013, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef]
- Miles, M.; Huberman, A.; Saldaña, J. Qualitative Data Analysis: A Methods Sourcebook, 2nd ed.; Sage Publications Ltd.: London, UK, 2014. [Google Scholar]
- Ayres, L.; Kavanaugh, K.; Knafl, K.A. Within-Case and Across-Case Approaches to Qualitative Data Analysis. Qual. Health Res. 2003, 13, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Clarke, R.M.; Jarrett, C.; Eckersberger, E.; Levine, Z.; Schulz, W.S.; Paterson, P. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.K.; Musa, M.S.; Tsiga-Ahmed, F.I.; Dayyab, F.M.; Sulaiman, A.K.; Bako, A.T. A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLoS ONE 2022, 17, e0278224. [Google Scholar] [CrossRef] [PubMed]
- Ojakaa, D.I.; Ofware, P.; Machira, Y.W.; Yamo, E.; Collymore, Y.; Ba-Nguz, A.; Vansadia, P.; Bingham, A. Community perceptions of malaria and vaccines in the South Coast and Busia regions of Kenya. Malar. J. 2011, 10, 147. [Google Scholar] [CrossRef]
- Bingham, A.; Gaspar, F.; Lancaster, K.; Conjera, J.; Collymore, Y.; Ba-Nguz, A. Community perceptions of malaria and vaccines in two districts of Mozambique. Malar. J. 2012, 11, 394. [Google Scholar] [CrossRef]
- Yaya Bocoum, F.; Kouanda, S.; Hinson, L.; Collymore, Y.; Ba-Nguz, A.; Bingham, A. Community perceptions of malaria vaccines: Qualitative research from the sanitary districts of Kaya and Hounde in Burkina Faso. Glob. Health Promot. 2014, 21, 76–87. [Google Scholar] [CrossRef]
- Meñaca, A.; Tagbor, H.; Adjei, R.; Bart-Plange, C.; Collymore, Y.; Ba-Nguz, A.; Mertes, K.; Bingham, A. Factors likely to affect community acceptance of a malaria vaccine in two districts of Ghana: A qualitative study. PLoS ONE 2014, 9, e109707. [Google Scholar] [CrossRef]
- Febir, L.G.; Asante, K.P.; Dzorgbo, D.-B.S.; Senah, K.A.; Letsa, T.S.; Owusu-Agyei, S. Community perceptions of a malaria vaccine in the Kintampo districts of Ghana. Malar. J. 2013, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.; Nderitu, D. Ethical considerations for introducing RTS,S/AS01 in countries with moderate to high Plasmodium falciparum malaria transmission. Lancet Glob. Health 2021, 9, e1642–e1643. [Google Scholar] [CrossRef]
- Eze, P.; Lawani, L.O.; Acharya, Y. Short message service (SMS) reminders for childhood immunisation in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e005035. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Framework for the Allocation of Limited Malaria Vaccine Supply. Available online: https://www.who.int/publications/m/item/framework-for-allocation-of-limited-malaria-vaccine-supply (accessed on 5 June 2023).
- SAGE/MPAG Working Group. Full evidence report on the RTS,S/AS01 malaria vaccine. In WHO Guidelines for Malaria: Systematic Reviews, Background Papers and Other Unpublished Evidence Considered in the Development of Recommendations; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. World Malaria Report; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. World Malaria Report 2017; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Atieli, H.E.; Zhou, G.; Afrane, Y.; Lee, M.C.; Mwanzo, I.; Githeko, A.K.; Yan, G. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasites Vectors 2011, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.C.; Hayden, M.H.; Olsen, H.; Cavanaugh, J.L.; Ruberto, I.; Agawo, M.; Munga, S. Comparing ownership and use of bed nets at two sites with differential malaria transmission in western Kenya. Malar. J. 2016, 15, 2016. [Google Scholar] [CrossRef] [PubMed]
- Pulford, J.; Hetzel, M.W.; Bryant, M.; Siba, P.M.; Mueller, I. Reported reasons for not using a mosquito net when one is available: A review of the published literature. Malar. J. 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- The malERA Refresh Consultative Panel on Insecticide and Drug Resistance. malERA: An Updated Research Agenda for Insecticide and Drug Resistance in Malaria Elimination and Eradication. PLoS Med. 2017, 14, e1002450. [Google Scholar]
- The RTS,S Clinical Trials Partnership. First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar] [CrossRef]
- The RTS,S Clinical Trials Partnership. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar] [CrossRef]
- Joint Technical Working Group and WHO Secretariat. Background Paper on the RTS,S/AS01 Malaria Vaccine; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Malaria Vaccine: WHO Position Paper; Weekly Epidemiological Record; WHO: Geneva, Switzerland, 2016; Volume 4, pp. 33–52. [Google Scholar]
- World Health Organization. Malaira Vaccine Implementation Programme (MVIP), Briefing Document; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- PATH Malaria Vaccine Initiative. Research on Community Perceptions of Vaccines and Malaria in Four African Countries; PATH Malaria Vaccine Initiative: Washington, DC, USA, 2014. [Google Scholar]
- Sallis, J.; Owen, N.; Fisher, E. Ecolgoical Models in Health Behavior. In Health Behavior and Education: Theory and Practice; Glanz, K., Rimer, B., Viswanath, K., Eds.; Jolley-Bass: San Francisco, CA, USA, 2008; pp. 464–485. [Google Scholar]
- Binghama, A.; Janmohamed, A.; Bartolini, R.; MCreed-Kanashir, H.; Ruhweza Katahoire, A.; Khan, I.; Lyazi, I.; Menezes, L.; Murokora, D.; Nguyen Quy, N.; et al. An Approach to Formative Research in HPV Vaccine Introduction Planning in Low-Resource Settings. Open Vaccine J. 2009, 2, 1–16. [Google Scholar] [CrossRef]
- PATH. Conducting Formative Research for HPV Vaccination Program Planning: Practical Experience from PATH; PATH: Seattle, WA, USA, 2012. [Google Scholar]
- Neale, B. What is Qualitative Longitudinal Research? forthcoming; Bloomsbury: London, UK, 2018. [Google Scholar]
- Leung, L. Validity, reliability, and generalizability in qualitative research. J. Fam. Med. Prim. Care 2015, 4, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.F.; Beck, C.T. Generalization in quantitative and qualitative research: Myths and strategies. Int. J. Nurs. Stud. 2010, 47, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Sandelowski, M.; Docherty, S.; Emden, C. Qualitative Metasynthesis: Issues and Techniques. Res. Nurs. Health 1997, 20, 365–371. [Google Scholar] [CrossRef]
- Schofield, J.W. Increasing the generalizability of qualitative research. In Qualitative Inquiry in Education: The Continuing Debate; Eisner, E., Peshkin, A., Eds.; Teachers College Press: New York, NY, USA, 1993; pp. 201–232. [Google Scholar]
- Barbour, R.S. Checklists for improving rigour in qualitative research: A case of the tail wagging the dog? Br. Med. J. 2001, 322, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Kitto, S.C.; Chesters, J.; Grbich, C. Quality in qualitative research: Criteria for authors and assessors in the submission and assessment of qualitative research articles for the Medical Journal of Australia. Med. J. Aust. 2008, 188, 243–246. [Google Scholar] [CrossRef]
- Meyrick, J. What is Good Qualitative Research? A First Step towrards a Comprehensive Approach to Judging Riquor/Quality. J. Health Psychol. 2006, 11, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm. Policy Ment. Health 2015, 42, 533–544. [Google Scholar] [CrossRef]
- Sandelowski, M. Sample Size in Qualitative Research. Res. Nurs. Health 1995, 18, 179–183. [Google Scholar] [CrossRef]
- Onwuegbuzie, A.J.; Collins, K.M. A Typology of Mixed Methods Sampling Designs in Social Science Research. Qual. Rep. 2007, 12, 281–316. [Google Scholar] [CrossRef]
- Kleinman, A. Patients and Healers in the Context of Culture: An Exploration of the Borderline between Anthropolog, Medicine, and Psychiatry; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
- Agar, M.H. The Professional Stranger: An Informal Introduction to Ethnography; Academic Press, Inc.: San Diego, CA, USA, 1980. [Google Scholar]
- Spradley, J.P. Participant Observation; Harcourt Brace Jovanovich College Publishers: New York, NY, USA, 1980. [Google Scholar]
- Maxwell, J.A. Using Qualitative Methods for Causal Explanation. Field Methods 2004, 16, 243–264. [Google Scholar] [CrossRef]
- Malterud, K. Systematic text condensation: A strategy for qualitative analysis. Scand. J. Public Health 2012, 40, 795–805. [Google Scholar] [CrossRef]
- Kvale, S. InterViews: Learning the Craft of Qualitative Research Interviewing, 3rd ed.; Sage Publications: Los Angeles, CA, USA, 2015. [Google Scholar]
| R1 | R2 | R3 | |
|---|---|---|---|
| Enrolled | 188 | 188 | 198 |
| Replaced | 0 | 10 | 0 |
| Total number enrolled | 188 | 198 | 198 |
| LTFU | 0 | −25 | −36 |
| Total number interviewed | 188 | 173 | 162 |
| Vaccination card not seen | −5 | −8 | −10 |
| Cases with complete data for uptake analysis | 183 | 165 | 152 |
| Characteristic | Number (%) | |||
|---|---|---|---|---|
| Ghana (n = 49, 13 LTFU) | Kenya (n = 55, 18 LTFU and 10 Replaced) | Malawi (n = 58, 5 LTFU) | ||
| Sex | Female | 47 (95.9) | 51 (92.7) | 58 (100.0) |
| Missing | -- | 1 (1.8) | -- | |
| Age (years) | 15–18 | 1 (2.0) | 1 (1.8) | -- |
| 19–24 | 10 (20.4) | 10 (18.1) | 21 (36.2) | |
| 25–29 | 13 (26.5) | 15 (27.2) | 14 (24.1) | |
| 30–34 | 14 (28.6) | 12 (21.8) | 10 (17.2) | |
| 35–40 | 8 (16.3) | 10 (18.1) | 10 (17.2) | |
| 40+ | 3 (6.1) | 6 (10.9) | 3 (5.1) | |
| Missing | -- | 1 (1.8) | -- | |
| Marital status | Married or cohabiting | 39 (79.6) | 52 (94.5) | 45 (77.5) |
| Divorced, widowed, unmarried | 10 (20.4) | 2 (3.6) | 13 (22.4) | |
| Missing | -- | 1 (1.8) | -- | |
| Education (highest completed) | None | 4 (8.2) | -- | 4 (6.8) |
| Primary | 9 (18.4) | 37 (67.2) | 45 (77.5) | |
| Secondary | 31 (63.3) | 13 (236) | 9 (15.5) | |
| Post-secondary | 5 (10.2) | 4 (7.2) | -- | |
| Number of children | 1 | 6 (12.2) | 4 (7.2) | 18 (31.0) |
| 2 | 12 (24.5) | 8 (14.5) | 12 (20.6) | |
| 3+ | 31 (63.3) | 42 (76.3) | 28 (48.2) | |
| Missing | -- | 1 (1.8) | -- | |
| Relation to child | Mother | 48 (98.0) | 50 (90.9) | 58 (100) |
| Grandparent or other | 1 (2.0) | 4 (7.2) | -- | |
| Missing | -- | 1 (1.8) | -- | |
| Data Completeness | RTS,S Doses Received | BCG, Penta, and Measles Immunization Status | ||||
|---|---|---|---|---|---|---|
| Doses Received | N | % of Total Enrolled (n = 198) | % of Cases w/Complete Data (n = 152) | Fully Immunized (n = 115/152) | Partially Immunized (n = 37/152) | |
| Complete (N = 152) | 4 | 98 | 49.5% | 64.5 | 87 | 11 |
| 3 | 34 | 17.2% | 22.4 | 20 | 14 | |
| 2 | 8 | 4.0% | 5.3 | 1 | 7 | |
| 1 | 1 | <1% | <1 | 0 | 1 | |
| 0 | 11 | 5.6% | 7.2 | 7 | 4 | |
| Incomplete (N = 46) | Card not seen | 10 | 5.1% | |||
| LTFU | 36 | 18.2% | ||||
| Total | 198 | 100% | ||||
| Category | Main Themes Discovered | |
|---|---|---|
| Facilitators | Impediments and Barriers | |
| Health System |
|
|
| Personal and Social |
|
|
| Information and Knowledge |
|
|
| Perceptions and Attitudes |
|
|
| Country and ID | Row | Service (Access, Availability) | Personal (Constraint, Attitude) | Information (Awareness, Knowledge) | |
|---|---|---|---|---|---|
| Ghana | C4_004 | 1 | Traveling; away from home | ||
| C5_004 | 2 | Unaware of 4th dose | |||
| C6_004 | 3 | Clinic too far away | “I don’t plan on taking her” (No time) | ||
| C6_005 | 4 | Traveling during RTS,S-4 visit | “I think the 3 doses are protecting her” | ||
| Kenya | C13_002 | 5 | “I was pregnant and very tired” | ||
| C13_004 | 6 | RTS,S-4 withheld; child being treated | |||
| C14_007 | 7 | Health worker strike, stockout | |||
| C15_003R | 8 | Health worker strike, stockout | Limited mobility (grandmother) | Limited awareness of doses and schedule | |
| C16_003 | 9 | With new baby: “I was kind of tired” | |||
| C16_004 | 10 | RTS,S-4 withheld; child being treated | |||
| C17_007 | 11 | Health worker strike | Has not returned since strike ended | ||
| C18_002 | 12 | Health worker strike | With the strike, “I developed some negligence” | ||
| C18_005 | 13 | Health worker strike | Has not returned since strike ended | ||
| C18_007 | 14 | “I’m remaining with one; have been negligent” | |||
| Malawi | C20_009 | 15 | COVID closure, then discouraged | Confused about number of doses | |
| C20_010 | 16 | Confused about doses and the number child has received | |||
| C21_015 | 17 | Has been sick and has competing demands | |||
| C22_028 | 18 | Turned away; schedule change | |||
| C23_029 | 19 | “As parents, we don’t count the doses” | Limited awareness of doses and schedule | ||
| C23_030 | 20 | Attends U5 clinic, assumes fully vaccinated | |||
| C23_031 | 21 | “I fell off” in those months. | Limited awareness of doses and schedule | ||
| C23_033 | 22 | “We don’t ask [or] count” | Surprised to learn there are four doses | ||
| C24_038 | 23 | Attends U5 clinic, assumes fully vaccinated | Limited awareness of doses and schedule | ||
| C24_040 | 24 | COVID closure | “I’ve been busy with planting season” | ||
| C25_049 | 25 | Too busy to attend last U5 clinic | Unaware that child is missing RTS,S-4 | ||
| C27_061 | 26 | COVID closure and record error | |||
| Case | Country | Key Adoption Barrier | Main Reason for Non-Adoption of RTS,S-1 | Immunization Status for Other Vaccines | ||
|---|---|---|---|---|---|---|
| Main Barrier | Reinforcing Barrier(s) | |||||
| Hesitancy | 1 | Ghana | Hesitant (RTS,S rumors) | Partner hesitant (RTS,S rumors) Info needs | Delayed acceptance | Missing BCG |
| 2 | Ghana | Hesitant (RTS,S rumors) | Partner hesitant (RTS,S rumors) | Delayed acceptance | Complete | |
| 3 | Ghana | Partner refusal (past AEFI) | Partner’s religious beliefs | Partner refusal | Complete | |
| 4 | Kenya | Hesitant (past AEFI) | RTS,S info needs Distance from facility Personal constraints | AEFI fears | Missing Penta3 and measles series | |
| Health System | 5 | Kenya | HW strike, stockouts | RTS,S info needs Poor HW attitudes | Service unavailable | Missing Penta3 and MR2 |
| 6 | Kenya | Stockouts | RTS,S info needs | Service unavailable | Complete | |
| 7 | Kenya | Distance from facility; uses private clinic closer to home | RTS,S info needs | Service inaccessible | Complete | |
| Info Needs | 8 | Malawi | RTS,S info needs | Fears (too many injections) | Unclear | Missing BCG |
| 9 | Malawi | RTS,S info needs | Poor HW attitudes | Believed child rcv’d some RTS,S doses | Missing Penta3 and measles series | |
| 10 | Malawi | RTS,S info needs | RTS,S info needs | Believed child rcv’d RTS,S-1 | Complete | |
| 11 | Malawi | RTS,S info needs | RTS,S info needs | Believed child rcv’d some RTS,S doses | Complete | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, J.; Gurley, N.; Gyapong, M.; Ansah, E.K.; Awusabo-Asare, K.; Gyasi, S.F.; Nkhoma, P.; Nyondo-Mipando, A.L.; Okello, G.; Webster, J.; et al. Acceptance of and Adherence to a Four-Dose RTS,S/AS01 Schedule: Findings from a Longitudinal Qualitative Evaluation Study for the Malaria Vaccine Implementation Programme. Vaccines 2023, 11, 1801. https://doi.org/10.3390/vaccines11121801
Price J, Gurley N, Gyapong M, Ansah EK, Awusabo-Asare K, Gyasi SF, Nkhoma P, Nyondo-Mipando AL, Okello G, Webster J, et al. Acceptance of and Adherence to a Four-Dose RTS,S/AS01 Schedule: Findings from a Longitudinal Qualitative Evaluation Study for the Malaria Vaccine Implementation Programme. Vaccines. 2023; 11(12):1801. https://doi.org/10.3390/vaccines11121801
Chicago/Turabian StylePrice, Jessica, Nikki Gurley, Margaret Gyapong, Evelyn Korkor Ansah, Kofi Awusabo-Asare, Samuel Fosu Gyasi, Pearson Nkhoma, Alinane Linda Nyondo-Mipando, George Okello, Jayne Webster, and et al. 2023. "Acceptance of and Adherence to a Four-Dose RTS,S/AS01 Schedule: Findings from a Longitudinal Qualitative Evaluation Study for the Malaria Vaccine Implementation Programme" Vaccines 11, no. 12: 1801. https://doi.org/10.3390/vaccines11121801
APA StylePrice, J., Gurley, N., Gyapong, M., Ansah, E. K., Awusabo-Asare, K., Gyasi, S. F., Nkhoma, P., Nyondo-Mipando, A. L., Okello, G., Webster, J., Desmond, N., Hill, J., & Gordon, W. S. (2023). Acceptance of and Adherence to a Four-Dose RTS,S/AS01 Schedule: Findings from a Longitudinal Qualitative Evaluation Study for the Malaria Vaccine Implementation Programme. Vaccines, 11(12), 1801. https://doi.org/10.3390/vaccines11121801






