Abstract
In countries with low tuberculosis (TB) incidence, the systematic testing and treatment of latent TB infection (LTBI) in contacts of pulmonary TB index cases is the standard of care. The objective of this study, conducted in Catalonia over 2019–2021, was to assess the factors associated with LTBI treatment prescription to close contacts of pulmonary TB index cases. In this population-based epidemiological study of LTBI prevalence among pulmonary TB contacts between 2019 and 2021, multiple logistic backward stepwise regression was used to identify the factors associated with treatment prescription, for which the adjusted odds ratio (aOR) and 95% confidence intervals (CI) were calculated. A total of 1487 LTBI contacts of 542 pulmonary TB index cases were studied, 80.6% of whom received a prescription. The factors associated with LTBI treatment prescription were exposure ≥6 h/day (aOR 14.20; 95% CI 5.22–38.66) and exposure <6 h/day (aOR 7.32, 95% CI 2.48–21.64), whereas the factors associated with no LTBI treatment prescription were age ≥55 years (aOR 0.22, 95% CI 0.08–0.64) and bacillus Calmette–Guerin vaccination (aOR 0.38, 95% CI 0.16–0.90). Crucial to LTBI treatment prescription is information on the contact’s duration of exposure to pulmonary TB, not only for contacts exposed for ≥6 h/day, but also for contacts with lower daily exposure levels.
1. Introduction
Tuberculosis (TB) is a major cause of morbidity and death worldwide. According to a 2022 World Health Organization (WHO) report, TB deaths in 2021 were 1.4 million and 187,000 among human immunodeficiency virus (HIV)-negative and HIV-positive individuals, respectively [1]. The WHO End TB Strategy aim is to achieve a 50% reduction in TB incidence by 2025 [2].
Latent TB infection (LTBI) is when, in the absence of active TB, a persistent immune response to Mycobacterium tuberculosis antigens can be demonstrated. Of individuals with LTBI, approximately 10% will develop active TB, half within the early years following infection [3,4]. The fact that one quarter of the world’s population is estimated to have LTBI [5] represents an important reservoir for future active TB cases, especially in risk groups such as the elderly, children, and HIV-positive and other immunosuppressed patients.
Adult and child contacts of pulmonary TB cases are systematically tested and treated for LTBI in countries with low TB incidence [6,7,8], and the recommendation is that treatment should be initiated and completed by at least 85% and 75% of infected contacts, respectively [9]. Nonetheless, the number of household contacts diagnosed with LTBI who are prescribed preventive treatment remains low [1]. Identifying and treating individuals with LTBI is crucial to preventing active TB development and transmission [10].
In Catalonia, a region in northeast Spain with 7.8 million inhabitants, the annual active TB incidence rates per 100,000 inhabitants were 14.1 in 2019 [11], 10.7 in 2020 [12], and 12.5 in 2021 [13], and the corresponding pulmonary disease rates were 64.4%, 66.6%, and 69.8%, respectively. According to the recommendations of the Generalitat of Catalonia’s Department of Health, the tracing of pulmonary TB index case contacts should aim to reduce morbidity and mortality among new cases of TB, detect sources of infection to reduce M. tuberculosis transmission, and contribute to TB elimination by treating new infections [14].
The aim of our study was to identify the factors associated with LTBI treatment prescription to close contacts of pulmonary TB index cases in Catalonia over a 30-month period in 2019–2021.
2. Materials and Methods
We performed a population-based epidemiological study of LTBI prevalence for all contacts of pulmonary TB cases in Catalonia between 2019 and 2021. The study population consisted of contacts of all new active pulmonary TB cases registered by the Catalan Epidemiological Surveillance Network (attached to the Public Health Agency of Catalonia) in the 30-month period from 1 January 2019 to 30 June 2021. Included in the study were contacts (household, school, workplace, recreational and other indoor settings) of pulmonary TB patients resident in Catalonia. The infectiousness period of the index case was determined to identify contacts, who were considered infected if they had a positive interferon gamma release assay (IGRA) or a tuberculin skin test (TST) induration diameter of ≥5 mm. Contacts underwent a clinical examination and posterior-anterior chest X-ray to rule out active TB.
Catalan Epidemiological Surveillance Network staff carried out an epidemiological survey of cases of pulmonary TB and enlisted household and community contacts by interviewing TB patients and healthcare workers who had reported active TB cases. Contacts were evaluated as soon as possible after index cases were diagnosed, especially if they showed sputum-smear positivity, and within 10–12 weeks after the last index case contact during the infectiousness period.
Registered contacts without active TB were administered the Catalan Epidemiological Surveillance Network [14] contact study questionnaire which was adapted to meet the objectives of the study. After evaluation in December 2018 in a pilot study of 50 contacts, it was considered that no additional changes to the questionnaire were necessary. The questionnaire compiled information on age, sex, migrant status, exposure setting (household, workplace, school, recreational and other), exposure duration (≥6 h/day, <6 h/day, non-daily, sporadic), tobacco use (smoker, ex-smoker, non-smoker), alcohol use, a history of bacillus Calmette–Guerin (BCG) vaccination, HIV, or immunosuppression, and TST or IGRA results. The information collected for pulmonary TB index cases of the studied contacts was as follows: sex, age, migrant status, chest X-ray results, bacteriology results, social vulnerability, HIV infection, tobacco use, alcohol use, and injecting-drug use (IDU). For contacts aged ≤13 years, the tobacco and alcohol variables were marked ‘no’ when data were missing.
Because Catalan treatment prescription guidelines [14] distinguish between contacts aged 0–5 and ≥6 years, we analyzed data overall and also separately for those two age brackets. The odds ratio (OR) and 95% confidence interval (CI) values were calculated to determine the associations between the dependent variable (treatment prescription) and the independent variables. To detect factors independently associated with treatment prescription, multiple logistic regression, performed using the backward stepwise method, included sex and all variables with p < 0.2, and the adjusted OR (aOR) and 95% CI values were calculated.
3. Results
3.1. Characteristics of Infected Contacts
A total of 1487 infected contacts of 542 pulmonary TB cases were studied. Figure 1 shows the geographical distribution of the infected contacts according to the Catalonia Epidemiological Surveillance Service (ESS) to which they belong. Most of the infected contacts (33.2%) belonged to the ESS of Barcelona City.
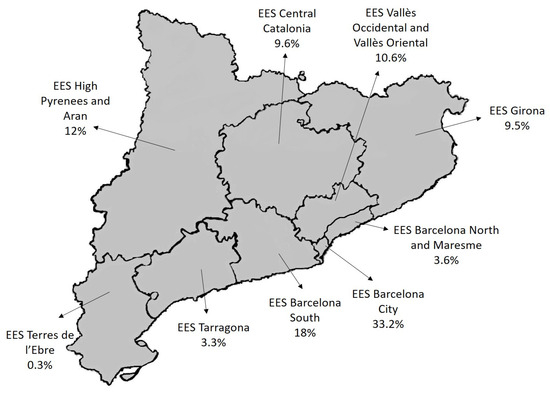
Figure 1.
Geographical distribution of the infected contacts.
Of the 1487 contacts, 642 were migrants and 443 had received the BCG vaccination. Only 64% of the vaccinated contacts had been tested for IGRA. LTBI treatment was prescribed to 80.6% of contacts, with no differences observed by sex (80.6% male and 80.6% female), neither overall nor considering only contacts aged ≥6 years (79.9% male and 79.8% female).
Table 1, which reports bivariate analysis results for contacts, shows that LTBI treatment was more frequently prescribed to contacts aged 0–5 years (OR 3.86; 95% CI 1.17–12.70) and 6–17 years (OR 2.44, 95% CI 1.39–4.27) than to contacts aged 35–54 years (OR 0.70, 95% CI 0.50–0.98) or ≥55 years (OR 0.39, 95% CI 0.27–0.57). Other factors associated with treatment prescription were household contact with the index case, exposure ≥6 h/day to the index case, tobacco use, and a TST induration diameter measuring 16–20.9 mm or 21–30.9 mm. The factors associated with no treatment prescription were workplace contact with the index case and BCG vaccination.

Table 1.
Characteristics of infected contacts of pulmonary TB index cases according to treatment prescription.
3.2. Characteristics of Pulmonary TB Index Cases
Table 2 summarizes details for the 542 pulmonary TB index cases for whom infected contacts were traced. The only factors associated with treatment prescription were acid-fast-stain (AFS) positivity (overall: OR 2.16, 95% CI 1.11–4.18; ≥6 years: OR 2.10, 95% CI 1.08–4.08) and HIV positivity (overall: OR 3.52, 95% CI 1.35–9.20; ≥6 years: OR 3.36, 95% CI 1.29–8.80).

Table 2.
Characteristics of pulmonary TB index cases whose infected contacts were analyzed according to treatment prescription.
3.3. Factors Associated with Treatment Prescription to Infected Contacts
Figure 2 reports the multivariate analysis results regarding the associations between the studied variables and LTBI treatment prescription. The factors associated with LTBI treatment prescription were exposure of ≥6 h/day (overall: aOR 14.20; 95% CI 5.22–38.66; ≥6 years: aOR 15.83, 95% CI 5.49–45.64), <6 h/day (overall: aOR 7.32, 95% CI 2.48–21.64; ≥6 years: aOR 7.52, 95% CI 2.79–22.74), and, only for contacts aged ≥6 years, migrant status (aOR 2.66, 95% CI 1.04–6.80). The factors associated with no treatment prescription were age ≥55 years (aOR 0.22, 95% CI 0.08–0.64) and BCG vaccination (overall: aOR 0.38, 95% CI 0.16–0.90; ≥6 years: aOR 0.40, 95% CI 0.17–0.98).
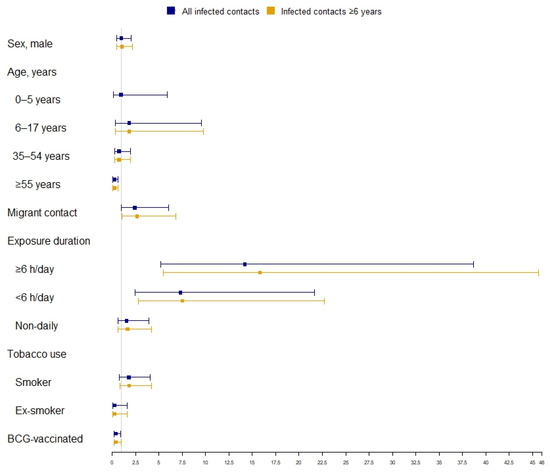
Figure 2.
Factors associated with treatment prescription to infected contacts of pulmonary TB index cases.
4. Discussion
LTBI treatment prescription after a medical evaluation of the contacts exposed to active TB cases is crucial to TB prevention [15,16]. In our study, LTBI treatment was prescribed to 80.6% of pulmonary TB contacts (79.9% of contacts ≥6 years), a proportion similar to the 80.2% reported by Sullivan et al. [17] in a study carried out at a community health center catering to a large non-US-born population.
Age was an important factor in decisions regarding LTBI treatment prescription. The bivariate analysis revealed that treatment was more frequently prescribed to children and adolescents (aged 0–5 and 6–17 years) and less frequently prescribed to individuals aged 35 years and older, a result that corroborates that reported for Australia by Dobler et al. [18]. In one US study by Tang et al. [19] carried out at a community health center, LTBI treatment was most frequently prescribed to contacts aged 6–17 years and less frequently to contacts older than 50 years. However, our multivariate analysis pointed to a significant association between no treatment prescription only for the contact group aged ≥55 years.
LTBI treatment prescription was also associated with migrant contacts aged ≥6 years. LTBI treatment decisions regarding migrants from countries with high TB incidence continues to be challenging, as the rate of active TB development over the long term appears to be higher in migrants with detected TB infection [20,21]. While Tang et al. [19] reported no association between migrant status and LTBI treatment prescription, our results suggesting that Catalan physicians take migrant status into account when prescribing treatment corroborates findings for a study carried out among new migrants in Montreal [22].
A noteworthy result of our study was the strong association found for exposure duration to the pulmonary TB index case, not only for contacts exposed for ≥6 h/day, but also for contacts exposed for <6 h/day. Dobler et al. [18] reported that non-household contacts were less frequently prescribed treatment than household contacts, although no information was provided on the exposure duration for household contacts. In our study, we found that LTBI treatment prescription overall and to contacts aged ≥6 years exposed to the TB index case for <6 h/day was around seven-fold greater than for sporadic exposure.
In assessing risks and priorities in contact studies, one of the factors considered is exposure to the index case, with an exposure of ≥6 h/day considered the highest priority for treatment prescription [14]. In our study, LTBI contacts exposed for ≥6 h/day showed the highest association with treatment prescription (around 14 and 15 times greater overall and for contacts aged ≥6 years, respectively, than for sporadic exposure)—which seems entirely logical given the high exposure level. However, the fact that the exposure of <6 h/day was also highly associated with treatment prescription would point to accurate risk assessment in clinical practice in Catalonia. Once treatment is prescribed, it is also important to monitor adherence in contacts exposed for <6 h/day, bearing in mind that, as reported by Gullón et al. [23] for Spain, an exposure level of <6 h/day is significantly related to a failure to initiate treatment.
While tobacco use was more frequently associated with LTBI treatment prescription than non-tobacco use in our bivariate analysis, the association was not statistically significant in the multivariate analysis. Other authors have reported similar findings, i.e., no association [24], or bivariate analysis association but no adjusted analysis association [18]. In their study in Catalonia, Godoy et al. [25] reported an association between smoking and LTBI, but reported no data on the association with treatment prescription. As pointed out by several authors [26,27,28], once close contacts have been identified, the data on both TB risk and treatment non-adherence risk need to be recorded so that the treatment of infected contacts can be prioritized.
In contrast to the Richards et al. [22] finding (for Canada) of no association between BCG vaccination in contacts and LTBI treatment prescription, we found that BCG-vaccinated contacts were less likely to be prescribed LTBI treatment than non-vaccinated contacts. In contrast to the recommendations for the management of TB contacts with a history of BCG vaccination [7,14], 36% of vaccinated contacts in our study had not undergone an IGRA test.
Although more research is needed on this point, a possible explanation could be physician doubts about TB diagnosis in BCG-vaccinated contacts. As suggested by several authors, knowledge of physician attitudes to TB prevention may be useful in advancing the elimination of the disease [29,30].
An exhaustive literature search retrieved the articles cited in the discussion section, but identified no studies reporting data for other Spanish regions. TB treatment prescription guidelines [14], as applied in Catalonia and in all other regions, follow Spanish TB Prevention and Control Plan [31] standards.
The main limitation of our study was missing data, largely attributable to the fact that the study was partially carried out during the first 18 months of the COVID-19 pandemic. It is likely that the corresponding impact on human resources meant that the recovery of a high proportion of the missing data by Catalan Epidemiological Surveillance Network staff was affected. Nonetheless, the large volume of data collected and analyzed would suggest that our results are consistent. A strength of our study is its population-based nature, as the whole population of Catalonia was covered for the 30-month period from 1 January 2019 to 30 June 2021 (12 months before and 18 months during the COVID-19 pandemic).
5. Conclusions
Our data would suggest that the information on the exposure duration to pulmonary TB index cases is crucial to LTBI treatment prescription, and that this information is important not only for exposure of ≥6 h/day, but also for lower daily exposure levels. IGRA testing should be carried out in all BCG-vaccinated contacts. Further research is needed on treatment prescription and non-prescription to contacts aged ≥55 years. LTBI testing and treatment of pulmonary TB index case contacts, as a cornerstone of the WHO End TB Strategy, is an essential component in worldwide TB prevention and control and should be practiced by all TB control teams.
Recommendations:
- -
- All pulmonary TB index case contacts should be routinely tested for LTBI and treated as necessary by TB control teams.
- -
- IGRA testing should be carried out in all BCG-vaccinated contacts.
- -
- Information on the exposure duration to pulmonary TB index cases is crucial to LTBI treatment prescription.
- -
- Further research is needed on treatment prescription to contacts aged ≥55 years.
Author Contributions
Á.D., P.G. and I.B. designed the study; J.-P.M., N.S., Transmission of Tuberculosis in Catalonia (Spain) Working Group, D.T. and I.P. generated the data; N.S. and I.P. performed analyses; Á.D., N.S. and D.T. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CIBER de Epidemiología y Salud Pública (CIBERESP), Programa 2 (PREVICET); the Ministry of Science and Innovation, Institute of Health Carlos III (Grant number PI18/01751) and Fondo Europeo de Desarrollo Regional (FEDER-Una manera de hacer Europa); and the Catalan Agency for the Management of Grants for University (AGAUR Grant Number 2021/SGR 00702).
Institutional Review Board Statement
The study was approved (code CEIC-2049) by the Ethics Committee of the Arnau de Vilanova University Hospital (Lleida) and was conducted according to European regulations and Declaration of Helsinki principles. All subjects included in the study received detailed information on the study aims before recruitment.
Informed Consent Statement
All data were collected as part of routine public health surveillance activities according to the legal mandate of the Department of Health of Catalonia, authorized to receive, process, and temporarily store personal data in cases of infectious diseases [32]. All data were fully anonymized.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
Transmission of Tuberculosis in Catalonia (Spain) Working Group members: Miquel Alsedà, Irene Barrabeig, Monica Carol, Joan A Caylà, Laura Clotet, Ángela Domínguez, Gloria Ferrús, Núria Follia, Pere Godoy, Mireia Jané, Sofia Minguell, Joan-Pau Millet, Angels Orcau, Ignasi Parrón, Pere Plans, Miriam Ros, Maria Sabater, Maria-Rosa Sala, and Diana Toledo.
Conflicts of Interest
The authors declare no competing interest.
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- World Health Organization. WHO End TB Strategy 2015. Available online: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19 (accessed on 4 February 2023).
- Heymann, D.L. Control of Communicable Disease Manual, 21st ed.; American Public Health Association: Washington, DA, USA, 2022. [Google Scholar]
- Trauer, J.M.; Moyo, N.; Tay, E.L.; Dale, K.; Ragonnet, R.; McBryde, E.S.; Denholm, J.T. Risk of active tuberculosis in the five years following infection. Chest 2016, 149, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and metaanalysis. Eur. Respir. J. 2019, 54, 1900655. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Gutierrez, S.M.B.; Bruchfeld, J.; Burhan, E.; et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Resp. J. 2015, 46, 1563. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Riley, S.R.; Lee, L.C.; Coffey, C.P.; Wang, S.H.; Asher, G.N.; Berry, A.M.; Williams, N.; Balio, C.; Voisin, C.E.; et al. Screening for latent tuberculosis infection in adults updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2023, 329, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2015.
- Erkens, C.G.M.; Kamphorst, M.; Abubakar, I.; Bothamley, G.H.; Chemtob, D.; Haas, W.; Migliori, G.B.; Rieder, H.L.; Zellweger, J.P.; Lange, C. Tuberculosis contact investigation in low prevalence countries: A European consensus. Eur. Respir. J. 2010, 36, 925–949. [Google Scholar] [CrossRef]
- Reichler, M.R.; Khan, A.; Sterling, T.R.; Zhao, H.; Moran, J.; McAuley, J.; Bessler, P.; Mangura, B. Risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. Infect. Dis. 2018, 218, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Mendioroz, J.; Pequeño, S.; López, M. La tuberculosi a Catalunya l’any 2019; Subdirecció General de Vigilància i Resposta a Emergències de Salut Pública: Barcelona, Spain, 2021. [Google Scholar]
- López, M.; Martínez, H.; Pequeño, S.; Sicart, E. Situació Epidemiològica i Tendència de L’endèmia Tuberculosa a Catalunya, 2020; Subdirecció General de Vigilància i Resposta a Emergències de Salut Pública: Barcelona, Spain, 2022. [Google Scholar]
- López, M.; Martínez, H.; Pequeño, S.; Sicart, E. La Tuberculosi a Catalunya L’any 2021; Subdirecció General de Vigilància i Resposta a Emergències de Salut Pública: Barcelona, Spain, 2023. [Google Scholar]
- Barrabeig-Fabregat, I.; Clotet, L.; Godoy, P.; Mercè, R.; Orcau-Palau, A.; Parrón, I.; Rodés-Monegal, A.; Sabater, M.; Torra-Bastardas, R. Recomanacions per a la Realització D’estudis de Contactes de Malalts amb Tuberculosi a Catalunya; Secretaria de Salut Pública: Barcelona, Spain, 2016. [Google Scholar]
- Alsdurf, H.; Hill, P.C.; Matteelli, A.; Getahun, H.; Menzies, D. The cascade of care diagnosed and treatment of latent tuberculosis infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 1269–1278. [Google Scholar] [CrossRef]
- Dobler, C.C. Unwarranted prescription variations for treatment of latent tuberculosis infection. Lancet Infect. Dis. 2017, 17, 134. [Google Scholar] [CrossRef]
- Sullivan, K.; Pease, C.; Zwerling, A.; Mallick, R.; Van Dyk, D.; Mulpuru, S.; Allen, C.; Alsdurf, H.; Alvarez, G.G. Seven-year retrospective study understanding the latent TB infection treatment cascade of care among adults in a low incidence country. BMC Public Health 2021, 21, 964. [Google Scholar] [CrossRef]
- Dobler, C.C.; Luu, Q.; Marks, G.B. What patient factors predict physicians’ decision not to treat latent tuberculosis infection in tuberculosis contacts? PLoS ONE 2013, 8, e76552. [Google Scholar] [CrossRef]
- Tang, A.S.; Mochizuki, T.; Dong, Z.; Flood, J.; Katrak, S.S. Can primary care drive tuberculosis elimination? Increasing latent tuberculosis infection testing and treatment initiation at a community health center with a large non-U.S.-born population. J. Immigr. Minor. Health 2023, 25, 803–815. [Google Scholar] [CrossRef]
- Shlomi, D.; Galor, I.; More, A.; Oberman, B.; Fireman, L. Latent tuberculosis infection prevalence in second generation immigrants from high to low TB burden countries. Pulmonology 2023, 29, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Winje, B.A.; Groneng, G.M.; White, R.A.; Akre, P.; Aavitsland, P.; Heldal, E. Immigrant screening for latent tuberculosis infection: Numbers needed to test and treat, a Norwegian population-based cohort. BMJ Open 2019, 9, e023412. [Google Scholar] [CrossRef]
- Richards, B.; Kozak, R.; Brassard, P.; Menzies, D.; Schwartzman, K. Tuberculosis surveillance among new immigrants in Montreal. Int. J. Tuberc. Lung Dis. 2005, 9, 858–864. [Google Scholar] [PubMed]
- Gullón Blanco, J.A.; Rodrigo Sanz, T.; Álvarez Navascues, F.; Tabernero Huguet, E.; Sabría Mestres, J.; García García, J.M.; Working Group of the Integrated Tuberculosis and NMT Research Program PII-TB NMT. Latent tuberculosis infection treatment: Compliance and factors related with initiation. Arch. Bronconeumol. 2023, 59, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Sangma, V.S.C.; Jaggi, S.; Saini, V.; Aggarwal, D.; Kumar, P.; Chander, J. Prevalence of latent tuberculosis infection in the household contacts of pulmonary tuberculosis, time to treat. Monaldi Arch. Chest Dis. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Parrón, I.; Barrabeig, I.; Caylà, J.A.; Clotet, L.; Follia, N.; Carol, M.; Orcau, A.; Alsedà, M.; Ferrús, G.; et al. Impact of COVID-19 pandemic on contact tracing of patients with pulmonary tuberculosis. Eur. J. Public Health 2022, 32, 643–647. [Google Scholar] [CrossRef]
- Marks, S.M.; Taylor, Z.; Qualls, N.L.; Shrestha-Kuwahara, R.J.; Wilce, M.A.; Nguyen, C.H. Outcomes of contact investigations of infectious tuberculosis patients. Am. J. Respir. Crit. Care Med. 2000, 162, 2033–2038. [Google Scholar] [CrossRef]
- Stockbridge, E.L.; Miller, T.L.; Carlson, E.K.; Ho, C. Predictors of latent tuberculosis infection treatment completion in the US private sector: An analysis of administrative claims. BMC Public Health 2018, 18, 662. [Google Scholar] [CrossRef]
- Álvarez, G.G.; VanDyk, D.D.; Aaron, S.D.; Cameron, D.W.; Davies, N.; Stephen, N.; Mallick, R.; Momoli, F.; Moreau, K.; Obed, N.; et al. TAIMA (Stop) TB: The impact of a multifaceted TB awareness and door-to-door campaign in residential areas of high risk for TB in Iqaluit, Nunavut. PLoS ONE 2014, 9, e100975. [Google Scholar] [CrossRef]
- Han, S.S.; Lee, S.J.; Yim, J.J.; Song, J.H.; Kang, Y.A. Evaluation and treatment of latent tuberculosis infection among healthcare workers in Korea: A multicentre cohort analysis. PLoS ONE 2019, 4, e0222810. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lee, J.S.; Kim, Y. Healthcare worker’s acceptance of and adherence to latent tuberculosis treatment. Occup. Med. 2023, 73, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Grupo de trabajo Plan Prevención y Control de la Tuberculosis. Plan para la Prevención y Control de la Tuberculosis en España. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud; Ministerio de Sanidad, Consumo y Bienestar Social: Madrid, Spain, 2019. [Google Scholar]
- Decret 2013/2015, de 15 de setembre, pel qual es crea la Xarxa de Vigilància Epidemiològica i es regulen els sistemes de notificació de malalties de declaració obligatòria i els brots epidèmics. DOGC 2015, 6958, 1–19.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).