COVID-19 Antibody Seroconversion in Cancer Patients: Impact of Therapy Cessation—A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
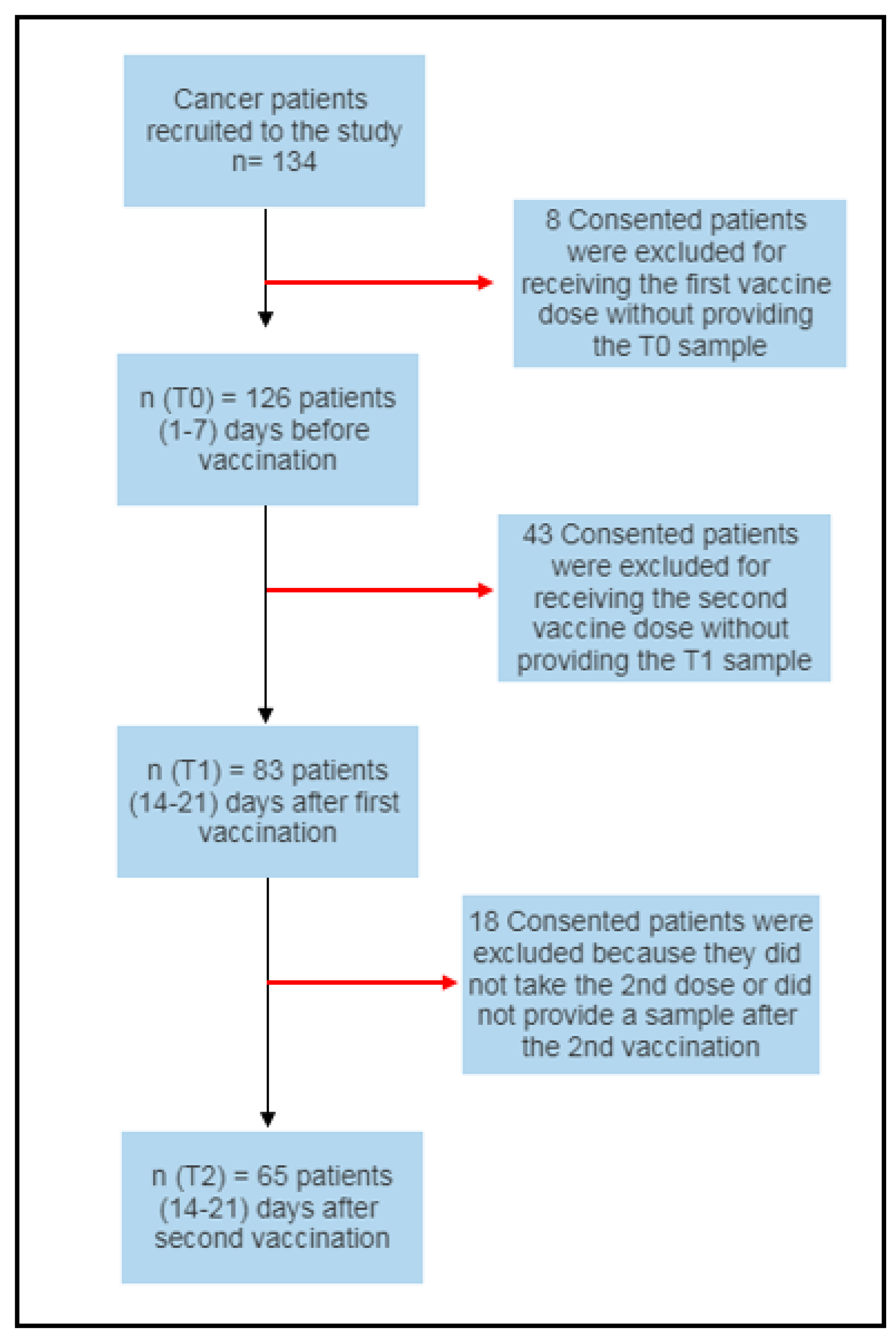
2.1.1. Study Participants
2.1.2. Vaccine Administration
- No symptoms
- Mild symptoms (e.g., mild fever, chills, or headache)
- Moderate symptoms (e.g., fevers above 38 °C and shivers that require medication)
- Severe symptoms leading to hospitalization.
2.1.3. Serological Assessment
2.2. Antibody Response Testing
2.3. Seroconversion and Breakthrough Cases
2.4. Statistical Analysis
3. Results
3.1. Patients’ Demographics
3.2. The Severity of Vaccine-Induced Side Effects after the First and Second Vaccine Doses
3.3. Evaluation of the Anti-COVID-19 Seroconversion Rate in Cancer Patients
3.4. Association of Seroconversion Rates with Cancer-Directed Therapy
3.5. Assess Cancer Patients’ Anti-COVID-19 Antibody Titer Responses with Gender
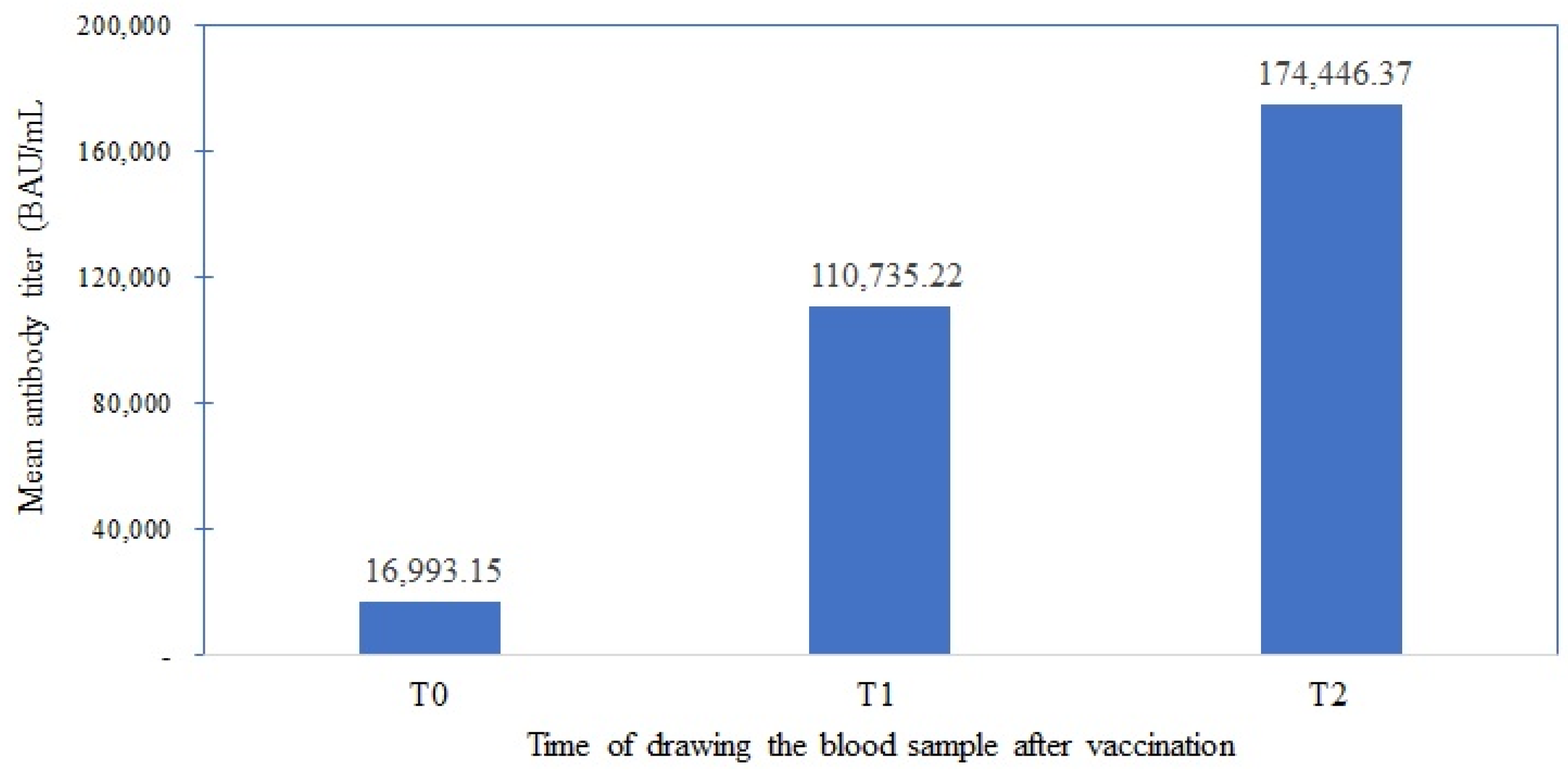
3.6. Assess Cancer Patients’ Anti-COVID-19 Antibody Titer Responses
3.7. SARS-CoV-2 Breakthrough Infection Rates in Cancer Patients after Each Vaccine Dose
3.8. Correlation Versus Agreement Levels of IgG Antibody Titer Measured by Roche and Abbott Systems over the Three Times Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Shah, M.K.; Hoyos, D.; Solovyov, A.; Douglas, M.; Taur, Y.; Maslak, P.; Babady, N.E.; Greenbaum, B.; Kamboj, M.; et al. Prolonged SARS-CoV-2 Infection in Patients with Lymphoid Malignancies. Cancer Discov. 2022, 12, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Shah, G.L.; Devlin, S.M.; Ramanathan, L.V.; Doddi, S.; Pessin, M.S.; Hoover, E.; Marcello, L.T.; Young, J.C.; Boutemine, S.R.; et al. Disease- and Therapy-Specific Impact on Humoral Immune Responses to COVID-19 Vaccination in Hematologic Malignancies. Blood Cancer Discov. 2021, 2, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Richter, J.; Rosenbaum, C.A.; Patel, D.; Smith, E.L.; Korde, N.; Lu, S.X.; Mailankody, S.; Shah, U.A.; Lesokhin, A.M.; et al. COVID-19 Infections and Clinical Outcomes in Patients with Multiple Myeloma in New York City: A Cohort Study from Five Academic Centers. Blood Cancer Discov. 2020, 1, 234–243. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2023; Available online: https://covid19.who.int/ (accessed on 23 October 2023).
- WHO. Jordan-WHO Coronavirus (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2023; Available online: https://covid19.who.int/region/emro/country/jo (accessed on 23 October 2023).
- WHO. WHO Issues Its First Emergency Use Validation for a COVID-19 Vaccine and Emphasizes Need for Equitable Global Access; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. WHO Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Jordan Launches a Vaccination Campaign against the Corona Virus [Press Release]; Arabia Sky News: Abu Dhabi, United Arab Emirates, 2021.
- Mariano, G.; Farthing, R.J.; Lale-Farjat, S.L.M.; Bergeron, J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020, 7, 605236. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Henderson, R.; Edwards, R.J.; Mansouri, K.; Janowska, K.; Stalls, V.; Gobeil, S.; Kopp, M.; Hsu, A.; Borgnia, M.; Parks, R.; et al. Controlling the SARS-CoV-2 Spike Glycoprotein Conformation. Nat. Struct. Mol. Biol. 2020, 27, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Shuja, S.H.; Asad, D.; Parekh, A.S. Sinopharm! An Unavoidable Contender in the Struggle against COVID. Infect. Drug Resist. 2021, 14, 3899–3900. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Ma, W.; Sikavi, D.R.; Drew, D.A.; Nguyen, L.H.; Bowyer, R.C.E.; Cardoso, M.J.; Fall, T.; Freidin, M.B.; Gomez, M.; et al. Cancer and Risk of COVID-19 Through a General Community Survey. Oncologist 2021, 26, e182–e185. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Bover, M.; Paredes, D.; Lopez-Lopez, F.; Jara-Casas, D.; Castelo-Loureiro, A.; Baena, J.; Mazarico, J.M.; Folgueira, M.D.; Meléndez-Carmona, M.Á.; et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: Analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer 2020, 135, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Kannenberg, J.; Biemann, R.; Hönemann, M.; Ackermann, G.; Jassoy, C. Comparison of the measured values of quantitative SARS-CoV-2 spike antibody assays. J. Clin. Virol. 2022, 155, 105269. [Google Scholar] [CrossRef]
- Roche, F.H.-L. Elecsys® Anti-SARS-CoV-2 S; F. Hoffmann-La Roche Ltd.: Basel, Switzerland, 2023. Available online: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html (accessed on 23 October 2023).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Leyfman, Y.; Emmanuel, N.; Menon, G.P.; Joshi, M.; Wilkerson, W.B.; Cappelli, J.; Erick, T.K.; Park, C.H.; Sharma, P. Cancer and COVID-19: Unravelling the immunological interplay with a review of promising therapies against severe SARS-CoV-2 for cancer patients. J. Hematol. Oncol. 2023, 16, 39. [Google Scholar] [CrossRef]
- Corti, C.; Crimini, E.; Tarantino, P.; Pravettoni, G.; Eggermont, A.M.M.; Delaloge, S.; Curigliano, G. SARS-CoV-2 vaccines for cancer patients: A call to action. Eur. J. Cancer 2021, 148, 316–327. [Google Scholar] [CrossRef]
- Kreuzberger, N.; Hirsch, C.; Andreas, M.; Böhm, L.; Bröckelmann, P.J.; Di Cristanziano, V.; Golinski, M.; ILona Hausinger, R.; Mellinghoff, F.; Lang, B.; et al. Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: A scoping review. Cochrane Database Syst. Rev. 2022, 8, Cd015021. [Google Scholar] [PubMed]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Barberi, V.; Maccallini, M.T.; Gariazzo, L.; Pontone, M.; Monti, A.; Campo, F.; Taraborelli, E.; et al. Immunogenicity and Safety of COVID-19 Vaccine BNT162b2 for Patients with Solid Cancer: A Large Cohort Prospective Study from a Single Institution. Clin. Cancer Res. 2021, 27, 6815–6823. [Google Scholar] [CrossRef]
- Mason, P.; Rizzuto, R.; Iannelli, L.; Baccaglini, F.; Rizzolo, V.; Baraldo, A.; Melloni, B.; Maffione, F.; Pezzoli, C.; Chiozza, M.L.; et al. Comparison of Adverse Effects of Two SARS-CoV-2 Vaccines Administered in Workers of the University of Padova. Vaccines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Matula, Z.; Gönczi, M.; Bekő, G.; Kádár, B.; Ajzner, É.; Uher, F.; Vályi-Nagy, I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccines 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Shin, H.M.; Choe, P.G.; Park, J.; Hong, J.; Seo, J.S.; Lee, Y.H.; Chang, E.; Kim, N.J.; Kim, M.; et al. Broad humoral and cellular immunity elicited by one-dose mRNA vaccination 18 months after SARS-CoV-2 infection. BMC Med. 2022, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Zirou, C.; Sklirou, A.D.; Apostolakou, F.; Gumeni, S.; Charitaki, I.; Papanagnou, E.D.; Bagratuni, T.; Liacos, C.I.; et al. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021, 19, 208. [Google Scholar] [CrossRef]
- Souan, L.; Sughayer, M.A.; Abualhour, M.M.; Siag, M.; Al-Badr, S.; Al-Atrash, T. Comparison of the Immunogenicity and Protective Efficacy of Various SARS-CoV-2 Vaccines among Healthcare Workers: Are Our White Coat Armies Protected? Vaccines 2022, 10, 642. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, S.; Liang, D.; Hu, W.; Ke, C.; He, J.; Yuan, R.; Huang, Y.; Li, Y.; Liu, D.; et al. Differential Antibody Response to Inactivated COVID-19 Vaccines in Healthy Subjects. Front. Cell Infect. Microbiol. 2021, 11, 791660. [Google Scholar] [CrossRef]
- Waldhorn, I.; Holland, R.; Goshen-Lago, T.; Shirman, Y.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Peer, A.; et al. Six-Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients with Solid Tumors. Cancer Discov. 2021, 11, 2430–2435. [Google Scholar] [CrossRef]
- Debie, Y.; Van Audenaerde, J.R.M.; Vandamme, T.; Croes, L.; Teuwen, L.-A.; Verbruggen, L.; Vanhoutte, G.; Marcq, E.; Verheggen, L.; Le Blon, D.; et al. Humoral and Cellular Immune Responses against SARS-CoV-2 after Third Dose BNT162b2 following Double-Dose Vaccination with BNT162b2 versus ChAdOx1 in Patients with Cancer. Clin. Cancer Res. 2023, 29, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Badano, M.N.; Sabbione, F.; Keitelman, I.; Pereson, M.; Aloisi, N.; Colado, A.; Ramos, M.V.; Ortiz Wilczyñski, J.M.; Pozner, R.G.; Castillo, L.; et al. Humoral response to the BBIBP-CorV vaccine over time in healthcare workers with or without exposure to SARS-CoV-2. Mol. Immunol. 2022, 143, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Clementi, N.; Criscuolo, E.; Ambrosi, A.; Corea, F.; Di Resta, C.; Tomaiuolo, R.; Mancini, N.; Locatelli, M.; Plebani, M.; et al. Antibody Titer Kinetics and SARS-CoV-2 Infections Six Months after Administration with the BNT162b2 Vaccine. Vaccines 2021, 9, 1357. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; Vazquez, L.; Calabuig, M.; López-Corral, L.; Martin-Martin, G.; Villalon, L.; Sanz-Linares, G.; Conesa-Garcia, V.; Sanchez-Salinas, A.; Gago, B.; et al. One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance evaluation of three automated quantitative immunoassays and their correlation with a surrogate virus neutralization test in coronavirus disease 19 patients and pre-pandemic controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Suemori, K.; Tanaka, K.; Okamoto, A.; Murakami, A.; Miyamoto, H.; Takasuka, Y.; Yamashita, M.; Takenaka, K. Long-term transition of antibody titers in healthcare workers following the first to fourth doses of mRNA COVID-19 vaccine: Comparison of two automated SARS-CoV-2 immunoassays. J. Infect. Chemother. 2023, 29, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Perkmann, T.; Mucher, P.; Perkmann-Nagele, N.; Radakovics, A.; Repl, M.; Koller, T.; Schmetterer, K.G.; Bigenzahn, J.W.; Leitner, F.; Jordakieva, G.; et al. The Comparability of Anti-Spike SARS-CoV-2 Antibody Tests is Time-Dependent: A Prospective Observational Study. Microbiol. Spectr. 2022, 10, e0140221. [Google Scholar] [CrossRef] [PubMed]
- Lukaszuk, K.; Kiewisz, J.; Rozanska, K.; Dabrowska, M.; Podolak, A.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, A.; Rabalski, L. Usefulness of IVD Kits for the Assessment of SARS-CoV-2 Antibodies to Evaluate the Humoral Response to Vaccination. Vaccines 2021, 9, 840. [Google Scholar] [CrossRef]
- Ivanov, A.; Kryshen, E.; Semenova, E. Nonlinear interdependence of the results of measuring anti-SARS-CoV-2 IgG levels using Abbott and Euroimmun test systems. J. Clin. Virol. 2023, 164, 105448. [Google Scholar] [CrossRef]
| Variables | Categories | n | % |
|---|---|---|---|
| Gender | Male | 11 | 16.9 |
| Female | 54 | 83.1 | |
| Cancer category | Solid cancer | 59 | 90.8 |
| Hematologic cancer | 6 | 9.2 | |
| Type of vaccine | BNT162B2 | 46 | 70.8 |
| BBIBP-CORV | 19 | 29.2 | |
| Age (years) | Mean ± SD | 53.2 ± 12.6 |
| Type of Active Cancer Therapy | n | % |
|---|---|---|
| No therapy | 22 | 33.8 |
| Hormonal Therapy | 8 | 12.3 |
| Immunotherapy | 1 | 1.5 |
| Radiotherapy | 1 | 1.5 |
| Chemotherapy ± other modalities * | 33 | 50.8 |
| Time Point | Vaccine Type | No Symptoms | Mild | Moderate Symptoms | Severe | p-Value |
|---|---|---|---|---|---|---|
| T1 | BNT162b2 (n = 46) | 14 (30.4%) | 24 (52.2%) | 7 (15.2%) | 1 (2.2%) | 0.222 |
| BBIBP-CorV (n = 19) | 9 (47.4%) | 10 (52.6%) | 0 (0%) | 0 (0%) | ||
| T2 | BNT162b2 (n = 46) | 18 (39.1%) | 23 (50%) | 5 (10.9%) | 0 (0%) | 0.209 |
| BBIBP-CorV (n = 19) | 8 (42.1%) | 10 (52.6%) | 0 (0%) | 1 (5.3%) |
| Vaccine | Time | Seroconversion Status | p-Value | ||
|---|---|---|---|---|---|
| BNT162b2 | T0 | Negative (n = 13) | Positive (n = 33) | ||
| T2 | Negative (n = 1) | 1 | 0 | <0.001 | |
| Positive (n = 45) | 12 | 33 | |||
| BBIBP-CorV | T0 | Negative (n = 8) | Positive (n = 11) | ||
| T2 | Negative (n = 2) | 2 | 0 | <0.031 | |
| Positive (n = 17) | 6 | 11 | |||
| Cancer-Directed Therapy | T1 | T2 | ||||
|---|---|---|---|---|---|---|
| Negative | Positive | p-Value | Negative | Positive | p-Value | |
| n (%) | n (%) | n (%) | n (%) | |||
| No therapy (n = 22) | 5 (22.7) | 17 (77.3) | 0.340 | 1 (4.5) | 21 (95.5) | 0.785 |
| Chemotherapy (n = 35) | 6 (17.1) | 29 (82.9) | 2 (5.7) | 33 (94.3) | ||
| Hormonal Therapy (n = 8) | 0.0 (0) | 8 (100.0) | 0.0 (0) | 8 (100.0) | ||
| Time Point | Vaccine Type | Breakthrough COVID-19 Infection | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| T1 | BNT162b2 | 41 (89.1%) | 5 (10.9%) | 0.310 |
| BBIBP-CorV | 19 (100%) | 0 (0%) | ||
| T2 | BNT162b2 | 43 (95.6%) | 2 (4.4%) | 0.341 |
| BBIBP-CorV | 17 (89.5%) | 2 (10.5%) | ||
| Roche BAU/mL | Abbott BAU/mL | Vaccine Type | Spearman Rho | p-Value |
|---|---|---|---|---|
| T0 | T0 | BNT162b2 BBIBP-CorV | 0.914 0.896 | <0.001 <0.001 |
| T1 | T1 | BNT162b2 BBIBP-CorV | 0.914 0.800 | <0.001 <0.001 |
| T2 | T2 | BNT162b2 BBIBP-CorV | 0.726 0.873 | <0.001 <0.001 |
| Measurement Times | ICC/Average Measure | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| T0 | 0.765 | 0.610 | 0.857 |
| T1 | 0.600 | 0.168 | 0.790 |
| T2 | 0.602 | 0.117 | 0.799 |
| Time Points | Cohen Kappa | p-Value | Interpretation |
|---|---|---|---|
| T0 | 0.598 | <0.001 | Moderate agreement |
| T1 | 0.616 | <0.001 | Substantial agreement |
| T2 | 0.024 | 0.825 | None to slight agreement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souan, L.; Abdel-Razeq, H.; Nashwan, S.; Al Badr, S.; Alrabi, K.; Sughayer, M.A. COVID-19 Antibody Seroconversion in Cancer Patients: Impact of Therapy Cessation—A Single-Center Study. Vaccines 2023, 11, 1659. https://doi.org/10.3390/vaccines11111659
Souan L, Abdel-Razeq H, Nashwan S, Al Badr S, Alrabi K, Sughayer MA. COVID-19 Antibody Seroconversion in Cancer Patients: Impact of Therapy Cessation—A Single-Center Study. Vaccines. 2023; 11(11):1659. https://doi.org/10.3390/vaccines11111659
Chicago/Turabian StyleSouan, Lina, Hikmat Abdel-Razeq, Sura Nashwan, Sara Al Badr, Kamal Alrabi, and Maher A. Sughayer. 2023. "COVID-19 Antibody Seroconversion in Cancer Patients: Impact of Therapy Cessation—A Single-Center Study" Vaccines 11, no. 11: 1659. https://doi.org/10.3390/vaccines11111659
APA StyleSouan, L., Abdel-Razeq, H., Nashwan, S., Al Badr, S., Alrabi, K., & Sughayer, M. A. (2023). COVID-19 Antibody Seroconversion in Cancer Patients: Impact of Therapy Cessation—A Single-Center Study. Vaccines, 11(11), 1659. https://doi.org/10.3390/vaccines11111659








