Factors Associated with Early Versus Late Uptake of the COVID-19 Vaccine during Pregnancy over Time in Australia: A Population-Based Cohort Study
Abstract
:1. Background
2. Methods
Analysis
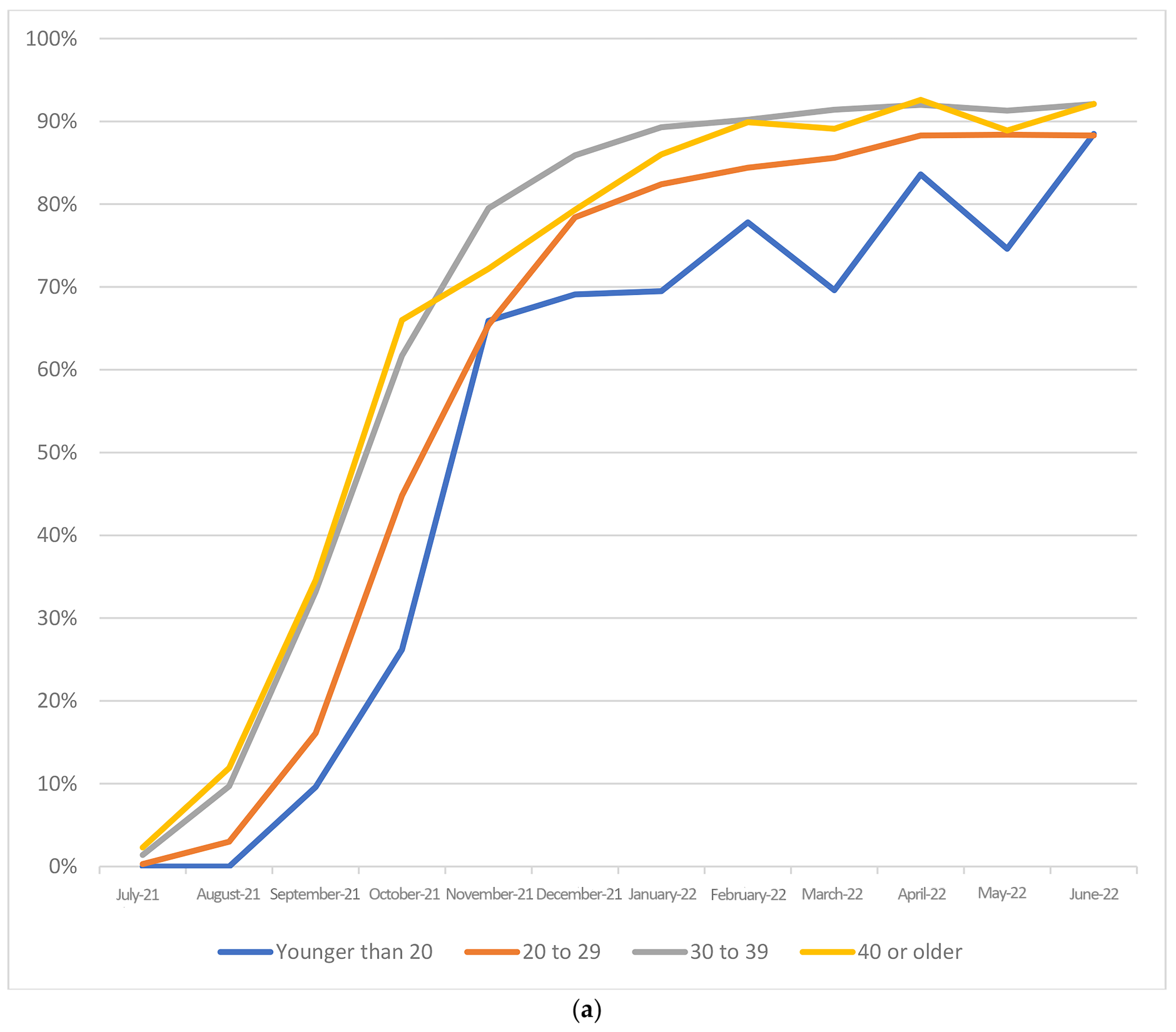
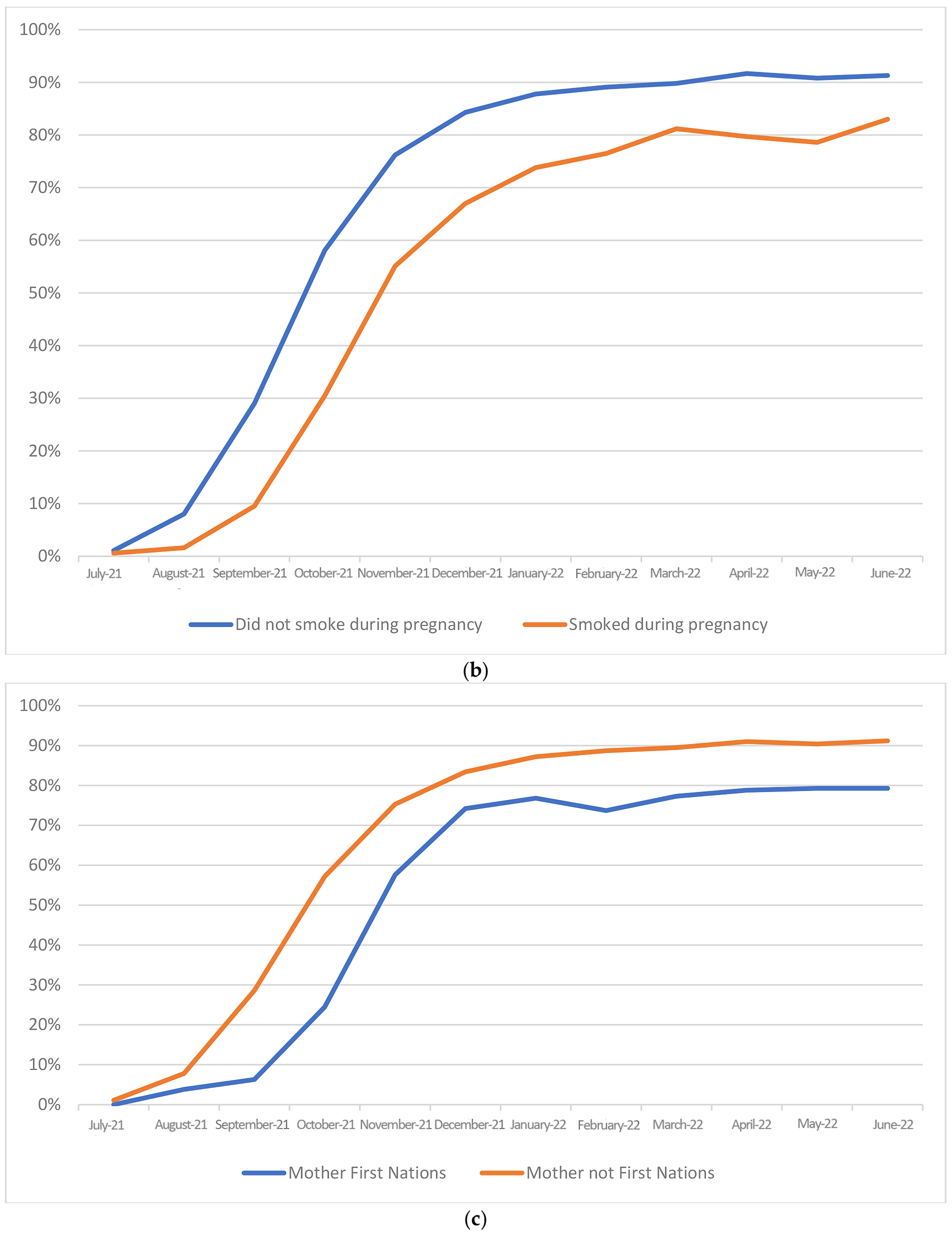
3. Results
4. Discussion
- Public health messages need to consider investing greater effort in younger women who may perceive themselves as “healthy”;
- Providers need to understand that women in their third or more pregnancy may need more information about “why” they are still recommended to receive vaccines when they may have received vaccines in prior pregnancies;
- Women with risk factors for respiratory illness such as smokers may need targeted messaging-, e.g., “if you are unable to stop smoking during pregnancy then there are measures you can consider to improve your health and that of your baby”;
- English proficiency, rather than country of birth, influences maternal vaccine uptake.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Health of Australia. First Confirmed Case of Novel Coronavirus in Australia. Available online: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/first-confirmed-case-of-novel-coronavirus-in-australia (accessed on 10 July 2023).
- Flanagan, K.L.; Best, E.; Crawford, N.W.; Giles, M.; Koirala, A.; Macartney, K.; Russell, F.; Teh, B.W.; Wen, S.C.H. Progress and Pitfalls in the Quest for Effective SARS-Co-V-2 (COVID-19) Vaccines. Front. Immunol. 2020, 11, 579250. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchen, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, C.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabediam, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–3 October 2020. Morb. Mortal Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Stallings, E.; Yap, M.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; De Marcillac, F.; Jouffrieau, C.; Kuhn, P.; Thuet, V.; Hansmann, Y.; Ruch, Y.; Fafi-Kremer, S.; Deruelle, P. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am. J. Obstet. Gynecol. 2020, 223, 914.e1–914.e15. [Google Scholar] [CrossRef] [PubMed]
- Australian Government, Department of Health and Aged Care. Joint Statement between RANZCOG and ATAGI about COVID-19 Vaccination for Pregnant Women. Available online: www.health.gov.au/news/joint-statement-between-ranzcog-and-atagi-about-covid-19-vaccination-for-pregnant-women (accessed on 10 July 2023).
- Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook; Australian Government Department of Health and Aged Care: Canberra, Australia, 2022. Available online: www.immunisationhandbook.health.gov.au (accessed on 10 July 2023).
- Victorian Government, Department of Health. Victorian Perinatal Data Collection (VPDC) Manual 2021–22, Sections 1–5a. 2021. Available online: https://www.health.vic.gov.au/publications/victorian-perinatal-data-collection-vpdcmanual-2021-22 (accessed on 10 July 2023).
- World Health Organisation. New Guidelines on Antenatal Care for a Positive Pregnancy Experience. 2016. Available online: https://www.who.int/news/item/07-11-2016-new-guidelines-on-antenatal-care-for-apositive-pregnancy-experience (accessed on 10 July 2023).
- Australian Government, Department of Health. Pregnancy Care Guidelines: Antenatal Visits. 2021. Available online: https://www.health.gov.au/resources/pregnancy-care-guidelines/part-b-core-practices-inpregnancy-care/antenatal-visits (accessed on 10 July 2023).
- McRae, J.E.; McHugh, L.; King, C.; Beard, F.H.; Blyth, C.C.; Danchin, M.H.; Giles, M.L.; Mohammed, H.; Wood, N.; Macartney, K. Influenza and pertussis vaccine coverage in pregnancy in Australia, 2016–2021. Med. J. Aust. 2023, 218, 11. [Google Scholar] [CrossRef] [PubMed]
- Australian Government, Department of Health. COVID-19 Vaccine Exemptions. Available online: https://www.health.gov.au/sites/default/files/documents/2022/02/general-covid-19-vaccine-exemptions-fact-sheet-covid-19-vaccine-exemptions_0.pdf (accessed on 10 July 2023).
- Rikard-Bell, M.; Elhindi, J.; Lam, J.; Seeho, S.; Black, K.; Melov, S.; Jenkins, G.; McNab, J.; Wiley, K.; Pasupathy, D. COVID-19 vaccine acceptance among pregnant women and the reasons for hesitancy: A multi-centre cross-sectional survey. Aust. N. Z. J. Obstet. Gynaecol. 2022, 63, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sohan, K.; Mohammed, Z.C.M.; Bachan, V. COVID-19 Vaccine Uptake, Acceptance, and Reasons for Vaccine Hesitancy: A Cross-Sectional Study Among Pregnant Women in Trinidad, West Indies. Int. J. Women’s Health 2023, 15, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.M.; D’Souza, G.; Bordner, C.; Allen, S.I.; Hobkirk, A.L.; Foulds, J.; Yingst, J.M. COVID-19 Vaccination Uptake and Hesitancy Among Current Tobacco Users. Tob. Use Insights 2021, 14, 1179173X211068027. [Google Scholar] [CrossRef] [PubMed]
- Australian Government, Department of Health and Aged Care. Australia’s COVID-19 Vaccine National Roll-Out Strategy. Available online: www.health.gov.au/sites/default/files/documents/2021/01/covide-19-vaccination-australia-s-covid-19-vaccine-national-roll-out-strategy.pdf (accessed on 10 July 2023).
- Graham, S.; Blaxland, M.; Bolt, R.; Beadman, M.; Gardner, K.; Martin, K.; Doyle, M.; Beetson, K.; Murphy, D.; Bell, S.; et al. Aboriginal people’s perspectives about COVID-19 vaccines and motivations to seek vaccination: A qualitative study. BMJ Global Health 2022, 7, e008815. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.L.; Davey, M.A.; Wallace, E.; Davey, M.A.; Wallace, E. Associations between maternal immunisation and reduced rates of preterm birth and stillbirth: A population based retrospective cohort study. Front. Immunol. 2021, 12, 704254. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.A.; Cook, R.E.; Button, J.A. Parent and Peer Norms are Unique Correlates of COVID-19 Vaccine Intentions in a Diverse Sample of U.S. Adolescents. J. Adolesc. Health 2021, 69, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ahmad, T.; Kazmi, T.; Sultan, F.; Afzal, S.; Safdar, R.M.; Khan, A.A. Community engagement to increase vaccine uptake: Quasi-experimental evidence from Islamabad and Rawalpindi, Pakistan. PLoS ONE 2022, 17, e0274718. [Google Scholar] [CrossRef] [PubMed]
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.A.; Failla, G.; Puleo, V.; Melnyk, A.; Lontano, A.; Ricciardi, W. Social media and attitudes towards a COVID-19 vaccination: A systematic review of the literature. EClinicalMedicine 2022, 48, 101454. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.L.; Khai, K.; Krishnaswamy, S.; Bellamy, K.; Angliss, M.; Smith, C.; Fay, O.; Paddle, P.; Vollenhoven, B. An evaluation of strategies to achieve greater than 90% coverage of maternal influenza and pertussis vaccines including an economic evaluation. BMC Pregnancy Childbirth 2021, 21, 771. [Google Scholar] [CrossRef] [PubMed]
- Baïssas, T.; Boisnard, F.; Cuesta Esteve, I.; Garcia Sánchez, M.; Jones, C.E.; Rigoine de Fougerolles, T.; Tan, L.; Vitoux, O.; Klein, C. Vaccination in pregnancy against pertussis and seasonal influenza: Key learnings and components from high-performing vaccine programmes in three countries: The United Kingdom, the United States and Spain. BMC Public Health 2021, 21, 2182. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. FDA Grants Breakthrough Therapy Designation to Pfizer’s Group B Streptococcus Vaccine Candidate to Help Prevent Infection in Infants via Immunization of Pregnant Women. News Release–Pfizer Inc. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-grants-breakthrough-therapy-designation-pfizers-group-b (accessed on 10 July 2023).
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.; Pahud, B.A.; Llapur, C.; Baker, J.; Perez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]

| YES (N = 42,281) | NO (N = 26,099) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | OR | 95%CI | aOR | 95%CI | |
| Hospital type | |||||||||
| Public | 36,119 | 62.7 | 21,478 | 37.3 | <0.001 | 0.57 | (0.55, 0.59) | 0.65 | (0.63, 0.68) |
| Private | 13,018 | 74.8 | 4390 | 25.2 | |||||
| Mother born in Australia | |||||||||
| Yes | 30,576 | 65.1 | 16,405 | 34.9 | 0.12 | 0.98 | (0.95, 1.01) | 1.03 | (1.00, 1.07) |
| No | 18,285 | 65.6 | 9571 | 34.4 | |||||
| Parity | |||||||||
| First birth | 22,566 | 67.4 | 10,913 | 32.6 | <0.001 | 1.42 | (1.37, 1.48) | 1.37 | (1.31, 1.43) |
| 2nd birth | 18,005 | 66.2 | 9198 | 33.8 | 1.35 | (1.29, 1.41) | 1.25 | (1.20, 1.31) | |
| 3rd or more | 8710 | 59.3 | 5988 | 40.7 | ref | ||||
| Gestation at first antenatal visit | |||||||||
| 4–13 weeks | 39,845 | 66.0 | 20,564 | 34.0 | <0.001 | ne | |||
| 14–27 weeks | 8394 | 63.7 | 4776 | 36.3 | |||||
| >27 weeks | 733 | 60.0 | 488 | 40.0 | |||||
| Number of antenatal visits | |||||||||
| <8 | 10,906 | 61.8 | 6746 | 38.2 | <0.001 | ||||
| >=8 | 37,975 | 66.4 | 19,229 | 33.6 | 1.22 | (1.18, 1.26) | 1.08 | (1.04, 1.12) | |
| Maternal smoking at any time during this pregnancy | |||||||||
| No | 44,156 | 66.1 | 22,660 | 33.9 | <0.001 | ||||
| Yes | 2835 | 52.2 | 2591 | 47.8 | 0.56 | (0.53, 0.59) | 0.7 | (0.66, 0.74) | |
| Maternal First Nations status | |||||||||
| Yes | 641 | 52.2 | 587 | 47.8 | 0.001 | 0.57 | (0.51, 0.63) | 0.83 | (0.74, 0.93) |
| No | 48,544 | 65.6 | 25,440 | 34.4 | |||||
| Received influenza vaccine during this pregnancy | |||||||||
| Yes | 35,969 | 67.2 | 17,567 | 32.8 | 0.001 | 1.33 | (1.28, 1.37) | 1.23 | (1.19, 1.28) |
| No | 12,749 | 60.7 | 8241 | 39.3 | |||||
| Received pertussis vaccine during this pregnancy | |||||||||
| Yes | 41,704 | 67.3 | 20,309 | 32.7 | 0.001 | 1.59 | (1.53, 1.65) | ne | |
| No | 7131 | 56.5 | 5495 | 43.5 | |||||
| Maternal age (years) | |||||||||
| Younger than 20 | 344 | 52.0 | 317 | 48.0 | <0.001 | 0.72 | (0.62, 0.84) | 0.81 | (0.69, 0.95) |
| 20–29 | 13,161 | 60.1 | 8763 | 40.0 | ref | ||||
| 30–39 | 33,100 | 67.8 | 15,701 | 32.2 | 1.4 | (1.36, 1.45) | 1.31 | (1.27, 1.36) | |
| 40 or older | 2675 | 67.1 | 1309 | 32.9 | 1.37 | (1.28, 1.47) | 1.29 | (1.20, 1.39) | |
| Discipline of main antenatal care provider | |||||||||
| Obstetrician | 30,141 | 67.5 | 14,502 | 32.5 | 0.001 | ne | |||
| Midwife | 15,524 | 62.8 | 9199 | 37.2 | |||||
| General practitioner | 3287 | 60.9 | 2114 | 39.1 | |||||
| None | 57 | 31.1 | 126 | 68.9 | |||||
| Other | 193 | 68.0 | 91 | 32.0 | |||||
| Maternal proficiency in spoken English * | |||||||||
| Very well | 15,074 | 67.7 | 7184 | 32.3 | <0.001 | ||||
| Well | 4988 | 66.3 | 2530 | 33.7 | |||||
| Not very well | 1435 | 62.6 | 857 | 37.4 | |||||
| Not at all | 454 | 61.8 | 281 | 38.2 | |||||
| Early Adopter | Late Adopter | Unvaccinated | Unclassifiable | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p-Value (All Groups) | (Early vs. Late Adopters | |
| Hospital type | ||||||||||
| Public | 32,527 | 75.4 | 2051 | 75.5 | 21,478 | 82.3 | 3208.0 | 56.0 | <0.001 | 0.796 |
| Private | 10,503 | 24.3 | 662 | 24.4 | 4389 | 16.8 | 2456.0 | 42.9 | ||
| Other | 108 | 0.3 | 5 | 0.2 | 231 | 0.9 | 61.0 | 1.1 | ||
| Mother born in Australia | ||||||||||
| Yes | 26,810 | 62.1 | 1613 | 59.4 | 16,405 | 62.9 | 3633.0 | 63.5 | <0.001 | 0.002 |
| No | 16,049 | 37.2 | 1074 | 39.5 | 9570 | 36.7 | 1986.0 | 34.7 | ||
| Not reported | 319 | 0.7 | 31 | 1.1 | 123 | 0.5 | 106.0 | 1.9 | ||
| Parity | ||||||||||
| First birth | 19,691 | 45.6 | 1288 | 47.4 | 10,913 | 41.8 | 2542.0 | 44.4 | <0.001 | 0.185 |
| 2nd birth | 15,804 | 36.6 | 968 | 35.6 | 9197 | 35.2 | 2039.0 | 35.6 | ||
| 3rd or more | 7683 | 17.8 | 462 | 17.0 | 5988 | 22.9 | 1144.0 | 20.0 | ||
| Gestation at first antenatal visit | ||||||||||
| 4–13 weeks | 34,880 | 80.8 | 2138 | 78.7 | 20,563 | 78.8 | 4693.0 | 82.0 | <0.001 | <0.001 |
| 14–27 weeks | 7466 | 17.3 | 481 | 17.7 | 4776 | 18.3 | 750.0 | 13.1 | ||
| >27 weeks | 577 | 1.3 | 76 | 2.8 | 488 | 1.9 | 160.0 | 2.8 | ||
| Not reported | 255 | 0.6 | 23 | 0.9 | 271 | 1.0 | 122.0 | 2.1 | ||
| Number of antenatal visits | ||||||||||
| <8 | 9549 | 22.1 | 678 | 24.9 | 6746 | 25.9 | 1383.0 | 24.2 | <0.001 | 0.002 |
| >=8 | 33,291 | 77.1 | 2022 | 74.4 | 19,232 | 73.7 | 4268.0 | 74.6 | ||
| Not reported | 338 | 0.8 | 18 | 0.7 | 120 | 0.5 | 74.0 | 1.3 | ||
| Maternal smoking at any time during this pregnancy | ||||||||||
| No | 38,714 | 89.7 | 2417 | 88.9 | 22,659 | 86.8 | 5076.0 | 88.7 | <0.001 | 0.001 |
| Yes | 2604 | 6.0 | 144 | 5.3 | 2630 | 10.1 | 339.0 | 5.9 | ||
| Not reported | 1860 | 4.3 | 157 | 5.8 | 809 | 3.1 | 310.0 | 5.4 | ||
| Maternal First Nations status | ||||||||||
| Yes | 568 | 1.3 | 46 | 1.7 | 598 | 2.3 | 88.0 | 1.5 | <0.001 | 0.243 |
| No | 42,540 | 98.5 | 2667 | 98.1 | 25,439 | 97.5 | 5609.0 | 98.0 | ||
| Not reported | 70 | 0.2 | 5 | 0.2 | 61 | 0.2 | 28.0 | 0.5 | ||
| Received influenza vaccine during this pregnancy | ||||||||||
| Yes | 31,590 | 73.2 | 1947 | 71.6 | 17,566 | 67.3 | 3784.0 | 66.1 | 0.001 | 0.192 |
| No | 11,122 | 25.8 | 733 | 27.0 | 8241 | 31.6 | 1666.0 | 29.1 | ||
| Not reported | 466 | 1.1 | 38 | 1.4 | 291 | 1.1 | 275.0 | 4.8 | ||
| Received pertussis vaccine during this pregnancy | ||||||||||
| Yes | 37,031 | 85.8 | 2345 | 86.3 | 20,309 | 77.8 | 3909.0 | 68.3 | 0.001 | 0.049 |
| No | 5795 | 13.4 | 339 | 12.5 | 5494 | 21.1 | 1531.0 | 26.7 | ||
| Not reported | 352 | 0.8 | 34 | 1.3 | 291 | 1.1 | 282.0 | 4.9 | ||
| Maternal age (years) | ||||||||||
| Younger than 20 | 307 | 0.7 | 19 | 0.7 | 317 | 1.2 | 45.0 | 0.8 | <0.001 | <0.001 |
| 20–29 | 11,787 | 27.3 | 699 | 25.7 | 8762 | 33.6 | 1413.0 | 24.7 | ||
| 30–39 | 28,891 | 66.9 | 1819 | 66.9 | 15,701 | 60.2 | 3818.0 | 66.7 | ||
| 40 or older | 2193 | 5.1 | 180 | 6.6 | 1309 | 5.0 | 447.0 | 7.8 | ||
| Not reported | - | 0.0 | <5 | 0.0 | 9 | 0.0 | <5 | 0.0 | ||
| Discipline of main antenatal care provider | ||||||||||
| Obstetrician | 25,781 | 59.7 | 1678 | 61.7 | 14,501 | 55.6 | 4173.0 | 72.9 | <0.001 | 0.043 |
| Midwife | 14,157 | 32.8 | 855 | 31.5 | 9199 | 35.3 | 1105.0 | 19.3 | ||
| General practitioner | 2976 | 6.9 | 165 | 6.1 | 2114 | 8.1 | 314.0 | 5.5 | ||
| None | 41 | 0.1 | 7 | 0.3 | 126 | 0.5 | 60.0 | 1.1 | ||
| Other | 151 | 0.4 | 10 | 0.4 | 91 | 0.4 | 44.0 | 0.8 | ||
| Not reported | 72 | 0.2 | 3 | 0.1 | 67 | 0.3 | 29.0 | 0.5 | ||
| Maternal proficiency in spoken English * | ||||||||||
| Very well | 13,167 | 30.5 | 940 | 34.6 | 7184 | 27.5 | 1535.0 | 26.8 | <0.001 | <0.001 |
| Well | 4299 | 10.0 | 277 | 10.2 | 2529 | 9.7 | 617.0 | 10.8 | ||
| Not very well | 1266 | 2.9 | 84 | 3.1 | 857 | 3.3 | 165.0 | 2.9 | ||
| Not at all | 408 | 0.9 | 31 | 1.1 | 281 | 1.1 | 64.0 | 1.1 | ||
| Not reported | 1063 | 2.5 | 72 | 2.7 | 381 | 1.5 | 191.0 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giles, M.L.; Krishnaswamy, S.; Coote, W.; Davey, M.-A. Factors Associated with Early Versus Late Uptake of the COVID-19 Vaccine during Pregnancy over Time in Australia: A Population-Based Cohort Study. Vaccines 2023, 11, 1713. https://doi.org/10.3390/vaccines11111713
Giles ML, Krishnaswamy S, Coote W, Davey M-A. Factors Associated with Early Versus Late Uptake of the COVID-19 Vaccine during Pregnancy over Time in Australia: A Population-Based Cohort Study. Vaccines. 2023; 11(11):1713. https://doi.org/10.3390/vaccines11111713
Chicago/Turabian StyleGiles, Michelle L., Sushena Krishnaswamy, William Coote, and Mary-Ann Davey. 2023. "Factors Associated with Early Versus Late Uptake of the COVID-19 Vaccine during Pregnancy over Time in Australia: A Population-Based Cohort Study" Vaccines 11, no. 11: 1713. https://doi.org/10.3390/vaccines11111713
APA StyleGiles, M. L., Krishnaswamy, S., Coote, W., & Davey, M.-A. (2023). Factors Associated with Early Versus Late Uptake of the COVID-19 Vaccine during Pregnancy over Time in Australia: A Population-Based Cohort Study. Vaccines, 11(11), 1713. https://doi.org/10.3390/vaccines11111713






