Trends in Influenza Vaccination Rates among a Medicaid Population from 2016 to 2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Population
2.3. Measures
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Vaccination Rates during Pre-COVID and Peri-COVID Era
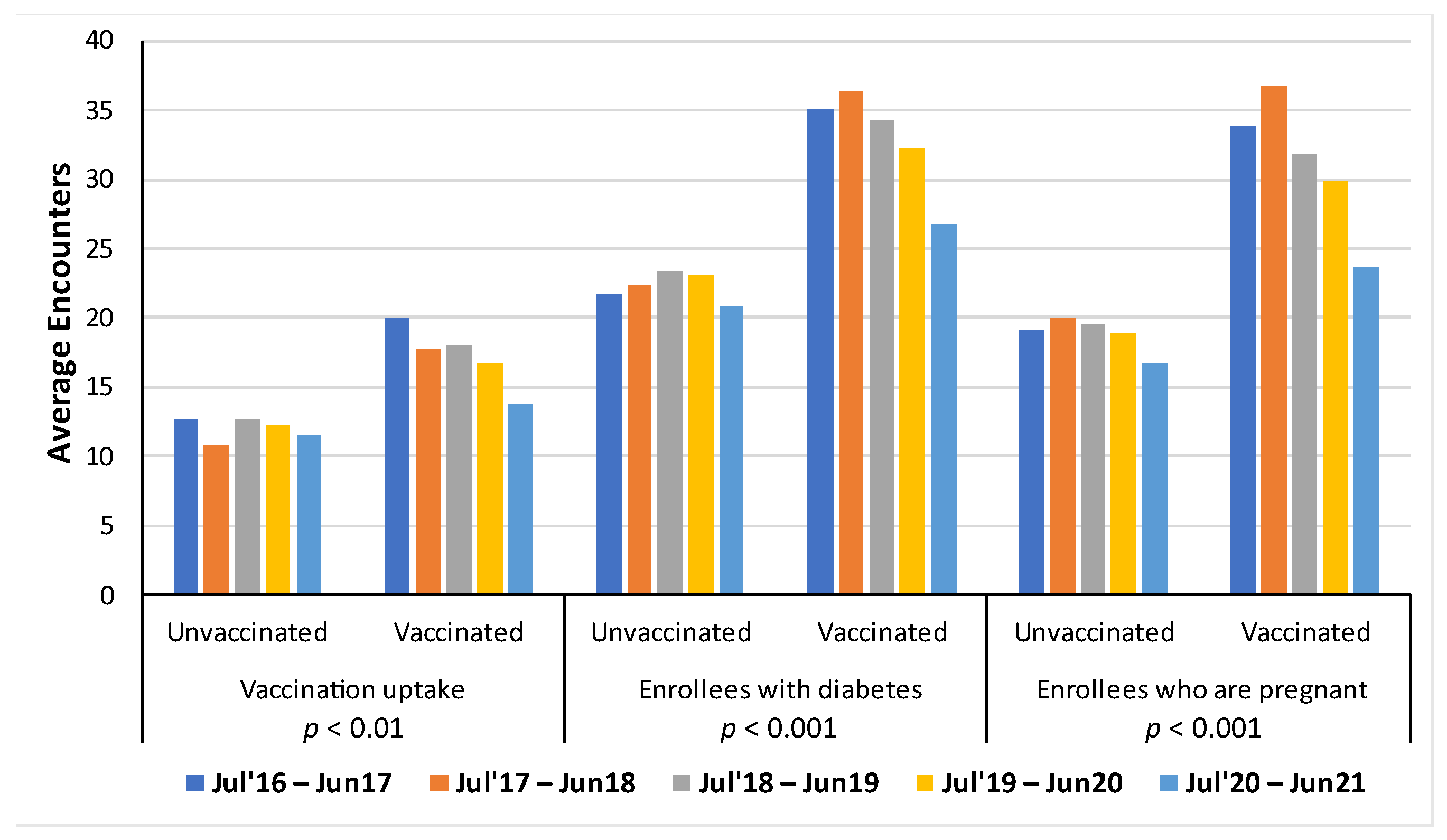
3.3. Average Number of In-Person Healthcare Encounters Per Influenza Seasons for Vaccinated, People with Diabetes, and Pregnant Women
3.4. Variables Associated with Influenza Vaccination Uptake
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, F.B. Provisional Mortality Data—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 597. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Murphy, S.; Kochanek, K.; Arias, E. Deaths: Final Data 2019. Natl. Vital Stat. Rep. 2021, 70, 8. Available online: https://stacks.cdc.gov/view/cdc/106058 (accessed on 26 July 2021).
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Past Seasons Estimated Influenza Disease Burden. Available online: https://www.cdc.gov/flu/about/burden/past-seasons.html (accessed on 1 October 2020).
- Putri, W.C.W.S.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Seasonal Flu Vaccines. Available online: https://www.cdc.gov/flu/prevent/flushot.htm (accessed on 25 August 2022).
- Medicaid and CHIP Payment and Access Commission. Vaccine Access for Adults Enrolled in Medicaid (Report to Congress on Medicaid and CHIP). Medicaid and CHIP Payment and Access Commission. 2022. Available online: https://www.macpac.gov/wp-content/uploads/2022/03/Chapter-2-Vaccine-Access-for-Adults-Enrolled-in-Medicaid.pdf (accessed on 31 March 2022).
- Centers for Disease Control and Prevention. People at Higher Risk of Flu Complications. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 25 August 2023).
- Tenforde, M.W.; Talbot, H.K.; Trabue, C.H.; Gaglani, M.; McNeal, T.M.; Monto, A.S.; Martin, E.T.; Zimmerman, R.K.; Silveira, F.P.; Middleton, D.B.; et al. Influenza Vaccine Effectiveness Against Hospitalization in the United States, 2019–2020. J. Infect. Dis. 2021, 224, 813–820. [Google Scholar] [CrossRef]
- Vamos, E.P.; Pape, U.J.; Curcin, V.; Harris, M.J.; Valabhji, J.; Majeed, A.; Millett, C. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ Can. Med. Assoc. J. 2016, 188, E342–E351. [Google Scholar] [CrossRef]
- Thompson, M.G.; Kwong, J.C.; Regan, A.K.; Katz, M.A.; Drews, S.J.; Azziz-Baumgartner, E.; Klein, N.P.; Chung, H.; Effler, P.V.; Feldman, B.S.; et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: A multi-country retrospective test negative design study, 2010–2016. Clin. Infect. Dis. 2019, 68, 1444–1453. [Google Scholar] [CrossRef]
- Benowitz, I.; Esposito, D.B.; Gracey, K.D.; Shapiro, E.D.; Vázquez, M. Influenza Vaccine Given to Pregnant Women Reduces Hospitalization Due to Influenza in Their Infants. Clin. Infect. Dis. 2010, 51, 1355–1361. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 Influenza Season. MMWR Recomm. Rep. 2019, 68, 1–21. [Google Scholar] [CrossRef]
- Medicaid.gov. Medicaid. Available online: https://www.medicaid.gov/medicaid/index.html (accessed on 31 July 2023).
- U.S. Department of Health and Human Services. Healthy People 2030: Increase the Proportion of People Who Get the Flu Vaccine Every Year (IID-09). Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-people-who-get-flu-vaccine-every-year-iid-09 (accessed on 27 October 2022).
- Medicaid.gov. May 2023 Medicaid & CHIP Enrollment Data Highlights. Available online: https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html (accessed on 22 September 2023).
- Dagenais, S.; Russo, L.; Madsen, A.; Webster, J.; Becnel, L. Use of Real-World Evidence to Drive Drug Development Strategy and Inform Clinical Trial Design. Clin. Pharmacol. Ther. 2022, 111, 77–89. [Google Scholar] [CrossRef]
- US Food and Drugs Administration. 2023; Real-World Evidence. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 5 February 2023).
- US Food and Drugs Administration. 2019; Sentinel System: Five-Year Strategy 2019–2023. Available online: https://www.fda.gov/media/120333/download (accessed on 31 January 2019).
- Eder, M.; Omic, H.; Gorges, J.; Badt, F.; Kikic, Z.; Saemann, M.D.; Tong, A.; Bauer, D.; Semmler, G.; Reiberger, T.; et al. Influenza vaccination uptake and factors influencing vaccination decision among patients with chronic kidney or liver disease. PLoS ONE 2021, 16, e0249785. [Google Scholar] [CrossRef]
- Harrison, N.; Poeppl, W.; Miksch, M.; Machold, K.; Kiener, H.; Aletaha, D.; Smolen, J.S.; Forstner, C.; Burgmann, H.; Lagler, H. Predictors for influenza vaccine acceptance among patients with inflammatory rheumatic diseases. Vaccine 2018, 36 Pt B, 4875–4879. [Google Scholar] [CrossRef]
- Payne, A.B.; Adamkiewicz, T.V.; Grosse, S.D.; Steffens, A.; Shay, D.K.; Reed, C.; Schieve, L.A. Influenza vaccination rates and hospitalizations among Medicaid enrollees with and without sickle cell disease, 2009–2015. Pediatr. Blood Cancer 2021, 68, e29351. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Influenza Vaccination Age Groups. Available online: https://www.cdc.gov/flu/fluvaxview/trends/age-groups.htm (accessed on 18 July 2012).
- Centers for Disease Control and Prevention. Influenza Vaccination: A Summary for Clinicians. Available online: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm (accessed on 31 August 2022).
- Healthcare Cost and Utilization Project (HCUP). Clinical Classifications Software Refined (CCSR). Agency for Healthcare Research and Quality. 2022. Available online: https://hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp (accessed on 12 December 2022).
- U.S. Department of Agriculture. Rural-Urban Continuum Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx (accessed on 10 December 2020).
- Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses, v2021.2. Available online: https://hcup-us.ahrq.gov/toolssoftware/ccsr/ccsr_archive.jsp#ccsr (accessed on 5 March 2021).
- Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2016–2017 Influenza Season. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm (accessed on 28 September 2017).
- Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2020–2021 Influenza Season. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-2021estimates.htm (accessed on 7 October 2021).
- McGovern, I.; Bogdanov, A.; Cappell, K.; Whipple, S.; Haag, M. Influenza Vaccine Uptake in the United States before and during the COVID-19 Pandemic. Vaccines 2022, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- The National Committee for Quality Assurance. 2023. Flu Vaccinations. Available online: https://www.ncqa.org/hedis/measures/flu-vaccinations/ (accessed on 31 August 2023).
- Blanford, E. Vaccination Disparities in the Medicaid Program. National Conference of State Legislatures. Available online: https://www.ncsl.org/news/details/vaccination-disparities-in-the-medicaid-program (accessed on 18 May 2022).
- Applewhite, A.; Stancampiano, F.F.; Harris, D.M.; Manaois, A.; Dimuna, J.; Glenn, J.; Heckman, M.G.; Brushaber, D.E.; Sher, T.; Valery, J.R. A Retrospective Analysis of Gender-Based Difference in Adherence to Influenza Vaccination during the 2018–2019 Season. J. Prim. Care Community Health 2020, 11, 2150132720958532. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.; Morgan, R.; Kuo, H.; Shea, P.; Shapiro, J.; Leng, S.X.; Pekosz, A.; Klein, S.L. Differences and disparities in seasonal influenza vaccine, acceptance, adverse reactions, and coverage by age, sex, gender, and race. Vaccine 2022, 40, 1643–1654. [Google Scholar] [CrossRef]
- Roy, M.; Sherrard, L.; Dubé, È.; Gilbert, N.L. Determinants of non-vaccination against seasonal influenza. Health Rep. 2018, 29, 12–22. [Google Scholar]
- Jain, B.; Paguio, J.A.; Yao, J.S.; Jain, U.; Dee, E.C.; Celi, L.A.; Ojikutu, B. Rural–Urban Differences in Influenza Vaccination Among Adults in the United States, 2018–2019. Am. J. Public Health 2022, 112, 304–307. [Google Scholar] [CrossRef]
- Zhai, Y.; Santibanez, T.A.; Kahn, K.E.; Srivastav, A.; Walker, T.Y.; Singleton, J.A. Rural, urban, and suburban differences in influenza vaccination coverage among children. Vaccine 2020, 38, 7596–7602. [Google Scholar] [CrossRef]
- Centers for Medicare and Medicaid. Adult and Child Health Care Quality Measures. 2023. Available online: https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/adult-and-child-health-care-quality-measures/index.html (accessed on 31 August 2023).
- Li, K.; Yu, T.; Seabury, S.A.; Dor, A. Trends and disparities in the utilization of influenza vaccines among commercially insured US adults during the COVID-19 pandemic. Vaccine 2022, 40, 2696–2704. [Google Scholar] [CrossRef]
- Roman, P.C. Influenza Vaccinations During the COVID-19 Pandemic—11 U.S. Jurisdictions, September–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1575. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.; Carr, K.; Danovaro-Holliday, M.C.; Sodha, S.V.; Prosperi, C.; Wunderlich, J.; Wonodi, C.; Reynolds, H.W.; Mirza, I.; Gacic-Dobo, M.; et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: Evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health 2022, 10, e186–e194. [Google Scholar] [CrossRef]
- Moll, K.; Wong, H.-L.; Fingar, K.; Zhou, C.K.; Lu, M.; Hu, M.; Hobbi, S.; Burrell, T.; Baer, B.; Simard, J.; et al. Vaccine exposure during pregnancy among privately and publicly insured women in the United States, 2016–2018. Vaccine 2021, 39, 6095–6103. [Google Scholar] [CrossRef]
- Cho, B.-H.; Weinbaum, C.; Tsai, Y.; Koppaka, R. Influenza Vaccine Uptake and Missed Opportunities among the Medicare-Covered Population with High-Risk Conditions during the 2018 to 2019 Influenza Season. Ann. Intern. Med. 2022, 175, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-C.; Lu, P.; Srivastav, A.; Cheng, Y.J.; Williams, W.W. Influenza vaccination coverage among adults with diabetes, United States, 2007–08 through 2017–18 seasons. Vaccine 2020, 38, 6545–6552. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-J.; O’Halloran, A.; Ding, H.; Srivastav, A.; Williams, W.W. Uptake of Influenza Vaccination and Missed Opportunities among Adults with High-Risk Conditions, United States, 2013. Am. J. Med. 2016, 129, e1–e636. [Google Scholar] [CrossRef] [PubMed]
- Poeppl, W.; Lagler, H.; Raderer, M.; Sperr, W.R.; Zielinski, C.; Herkner, H.; Burgmann, H. Influenza vaccination perception and coverage among patients with malignant disease. Vaccine 2015, 33, 1682–1687. [Google Scholar] [CrossRef]
- Druss, B.G.; Rask, K.; Katon, W.J. Major Depression, Depression Treatment, and Quality of Primary Medical Care. Gen. Hosp. Psychiatry 2008, 30, 20–25. [Google Scholar] [CrossRef]
- Druss, B.G.; Rosenheck, R.A.; Desai, M.M.; Perlin, J.B. Quality of Preventive Medical Care for Patients with Mental Disorders. Med. Care 2002, 40, 129. [Google Scholar] [CrossRef]
- Hassouneh, L.; Dunsiger, S. The impact of mental distress on influenza vaccine coverage. PLoS ONE 2022, 17, e0266692. [Google Scholar] [CrossRef]
- Lorenz, R.A.; Norris, M.M.; Norton, L.C.; Westrick, S.C. Factors Associated with Influenza Vaccination Decisions among Patients with Mental Illness. Int. J. Psychiatry Med. 2013, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y.P.S. Diabetes and psychiatric disorders. Indian J. Endocrinol. Metab. 2011, 15, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.W.; Lu, P.J.; O'Halloran, A.; Kim, D.K.; Grohskopf, L.A.; Pilishvili, T.; Skoff, T.H.; Nelson, N.P.; Harpaz, R.; Markowitz, L.E.; et al. Surveillance of Vaccination Coverage Among Adult Populations-United States, 2014. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2016, 65, 1–36. [Google Scholar] [CrossRef] [PubMed]


| Overall (July 2016–June 2021) | Pre-COVID (July 2016–June 2020) | Peri-COVID (July 2020–June 2021) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Total Enrollees | % | Vaccinated | Total Enrollees | % | Vaccinated | Total Enrollees | % | |
| Total | 3,050,471 | 20,868,910 | 15 | 2,407,644 | 16,807,523 | 14 | 642,827 | 4,061,387 | 16 |
| Age Groups | |||||||||
| 6 months–4 years | 734,672 | 2,565,290 | 29 | 562,144 | 2,007,435 | 28 | 172,528 | 557,855 | 31 |
| 5 years–17 years | 1,113,634 | 6,706,443 | 17 | 883,619 | 5,457,074 | 16 | 230,015 | 1,249,369 | 18 |
| 18 years–64 years | 1,202,165 | 11,597,177 | 10 | 961,881 | 9,343,014 | 10 | 240,284 | 2,254,163 | 11 |
| Gender | |||||||||
| Female | 1,714,621 | 11,596,733 | 15 | 1,353,614 | 9,308,460 | 15 | 361,007 | 2,288,273 | 16 |
| Male | 1,335,850 | 9,272,177 | 14 | 1,054,030 | 7,499,063 | 14 | 281,820 | 1,773,114 | 16 |
| Metropolitan Area | |||||||||
| Metropolitan | 2,452,070 | 16,686,225 | 15 | 1,924,884 | 13,357,713 | 14 | 527,186 | 3,328,512 | 16 |
| Non-Metropolitan | 598,401 | 4,182,685 | 14 | 482,760 | 3,449,810 | 14 | 115,641 | 732,875 | 16 |
| Education | |||||||||
| Greater than Median Higher Education Area | 1,380,567 | 9,410,850 | 15 | 1,088,778 | 7,567,529 | 14 | 291,789 | 1,843,321 | 16 |
| Lower than Median Higher Education Area | 1,669,904 | 11,458,060 | 15 | 1,318,866 | 9,239,994 | 14 | 351,038 | 2,218,066 | 16 |
| Medicaid Managed Care | |||||||||
| With Managed Care Program | 1,249,118 | 7,537,820 | 17 | 985,363 | 5,978,761 | 16 | 263,755 | 1,559,059 | 17 |
| Free-for-service | 1,801,353 | 13,331,090 | 14 | 1,422,281 | 10,828,762 | 13 | 379,072 | 2,502,328 | 15 |
| At-risk Health Conditions | |||||||||
| Diabetes * | 554,671 | 3,348,282 | 17 | 462,789 | 2,755,810 | 17 | 91,882 | 592,472 | 16 |
| Diabetes Type 1 (DM 1) | 103,442 | 542,915 | 19 | 87,351 | 448,292 | 19 | 16,091 | 94,623 | 17 |
| Diabetes Type 2 (DM 2) | 530,056 | 3,174,874 | 17 | 442,665 | 2,614,529 | 17 | 87,391 | 560,345 | 16 |
| Pregnancy | 593,094 | 3,423,662 | 17 | 442,533 | 2,558,276 | 17 | 33,184 | 203,434 | 16 |
| Overall (July 2016–June 2021) | Pre-COVID (July 2016–June 2020) | Peri COVID (July 2020–June 2021) | |
|---|---|---|---|
| Demographics Characteristics | |||
| 6 months–4 years (vs. 18–64 years) | 3.11 (3.09, 3.14) | 3.25 (3.22, 3.27) | 2.88 (2.85, 2.92) |
| 5–17 years (vs. 18–64 years) | 1.91 (1.9, 1.92) | 1.93 (1.92, 1.94) | 1.90 (1.88, 1.91) |
| Male (vs. Female) | 0.97 (0.97, 0.97) | 0.97 (0.97, 0.98) | 0.95 (0.94, 0.96) |
| Area with Higher than Median Education (vs. Not) | 1.00 (1, 1.01) | 1.01 (1, 1.01) | 1.00 (0.99, 1) |
| Metropolitan (vs. Non-Metropolitan) | 1.01 (1.01, 1.02) | 0.99 (0.98, 0.99) | 1.00 (1, 1.01) |
| Managed Care (vs. Non-Managed Care) | 1.18 (1.17, 1.18) | 1.21 (1.21, 1.22) | 1.10 (1.1, 1.11) |
| CCSR Diabetes Mapped Categories | |||
| Diabetes mellitus with complication | 1.06 (1.05–1.07) | 1.07 (1.07–1.08) | 1.04 (1.03–1.05) |
| Diabetes mellitus without complication | 1.01 (1.01–1.02) | 1.01 (1.00–1.01) | 1.02 (1.01–1.03) |
| CCSR Pregnancy Mapped Categories | |||
| Antenatal screening | 1.34 (1.33–1.36) | 1.29 (1.27–1.30) | 1.17 (1.15–1.19) |
| Malposition, disproportion, or other labor complications | 1.22 (1.20–1.23) | 1.18 (1.16–1.19) | 1.18 (1.16–1.21) |
| Supervision of high-risk pregnancy | 1.13 (1.19–1.14) | 1.12 (1.11–1.14) | 1.08 (1.06–1.10) |
| Other specified complications in pregnancy | 1.13 (1.11–1.14) | 1.10 (1.09–1.16) | 1.13 (1.11–1.15) |
| Maternal outcome of delivery | 1.12 (1.10–1.13) | 1.10 (1.08–1.11) | 1.01 (0.99–1.03) |
| Maternal care related to fetal conditions | 1.10 (1.09–1.12) | 1.08 (1.07–1.10) | 1.05 (1.03–1.07) |
| Uncomplicated pregnancy, delivery, or puerperium | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 0.97 (0.95–0.99) |
| Gestational weeks | 0.77 (0.76–0.78) | 0.80 (0.79–0.81) | 0.76 (0.75–0.77) |
| Top 10 CCSR Condition Categories | |||
| Contact with Health Services (Medical examination/evaluation) | 2.07 (2.06, 2.08) | 1.91 (1.9, 1.92) | 1.91 (1.89, 1.92) |
| Encounter for Perinatal Period (Liveborn) | 1.79 (1.77, 1.8) | 1.41 (1.4, 1.42) | 1.59 (1.57, 1.6) |
| Encounter for Pregnancy, Childbirth, and the Puerperium (Antenatal screening) | 1.34 (1.33, 1.36) | 1.29 (1.27, 1.3) | 1.17 (1.15, 1.19) |
| Contact with Health Services (Encounter for observation and examination, excludes infectious disease, neoplasm, mental disorders) | 1.29 (1.28, 1.29) | 1.24 (1.24, 1.25) | 1.22 (1.21, 1.22) |
| Contact with Health Services (Neoplasm-related encounters) | 1.27 (1.26, 1.27) | 1.25 (1.25, 1.26) | 1.25 (1.24, 1.26) |
| Contact with Health Services (Mental health conditions) | 1.26 (1.24, 1.27) | 1.10 (1.09, 1.11) | 1.41 (1.39, 1.42) |
| Encounter for Pregnancy, Childbirth, and the Puerperium (Labor complications) | 1.22 (1.2, 1.23) | 1.18 (1.16, 1.19) | 1.18 (1.16, 1.21) |
| Encounter for Eye and Adnexa (Refractive error) | 1.22 (1.21, 1.22) | 1.21 (1.21, 1.22) | 1.05 (1.04, 1.06) |
| Encounter for Eye and Adnexa (Cataract and other lens disorders) | 1.21 (1.2, 1.22) | 1.24 (1.22, 1.25) | 1.13 (1.11, 1.15) |
| Encounter for Respiratory system (Other specified upper respiratory infections) | 1.21 (1.2, 1.21) | 1.22 (1.21, 1.22) | 1.03 (1.02, 1.04) |
| Bottom 10 CCSR Condition Categories | |||
| Encounter for Circulatory System (Heart failure) | 0.90 (0.89, 0.91) | 0.88 (0.87, 0.89) | 0.92 (0.91, 0.94) |
| Encounter for Diseases of the Genitourinary System (Menopausal disorders) | 0.89 (0.88, 0.9) | 0.91 (0.9, 0.92) | 0.89 (0.87, 0.9) |
| Encounter for Nervous System Disorder (Epilepsy; convulsions) | 0.89 (0.88, 0.9) | 0.90 (0.9, 0.91) | 0.97 (0.95, 0.99) |
| Encounter for Mental, Behavioral, and Neurodevelopmental Disorders (Tobacco-related disorders) | 0.89 (0.88, 0.9) | 0.90 (0.9, 0.91) | 0.89 (0.87, 0.9) |
| Encounter for Mental, Behavioral, and Neurodevelopmental Disorders (Bipolar and related disorders) | 0.87 (0.87, 0.88) | 0.88 (0.87, 0.89) | 0.85 (0.84, 0.86) |
| Encounter for Mental, Behavioral, and Neurodevelopmental Disorders (Schizophrenia spectrum and other psychotic disorders) | 0.87 (0.86, 0.88) | 0.89 (0.88, 0.9) | 0.90 (0.89, 0.91) |
| Encounter for Mental, Behavioral, and Neurodevelopmental Disorders (Opioid-related disorders) | 0.84 (0.83, 0.85) | 0.87 (0.86, 0.88) | 0.84 (0.83, 0.85) |
| Encounter for Diseases of the Genitourinary System (Inflammatory diseases of female pelvic organs) | 0.83 (0.83, 0.84) | 0.85 (0.85, 0.86) | 0.76 (0.75, 0.77) |
| Encounter for Pregnancy, Childbirth, and the Puerperium (Gestational weeks) | 0.83 (0.82, 0.84) | 0.85 (0.84, 0.85) | 0.84 (0.83, 0.85) |
| Contact with Health Services (No immunization or under immunization) | 0.62 (0.61, 0.62) | 0.65 (0.64, 0.65) | 0.51 (0.5, 0.52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naderalvojoud, B.; Shah, N.D.; Mutanga, J.N.; Belov, A.; Staiger, R.; Chen, J.H.; Whitaker, B.; Hernandez-Boussard, T. Trends in Influenza Vaccination Rates among a Medicaid Population from 2016 to 2021. Vaccines 2023, 11, 1712. https://doi.org/10.3390/vaccines11111712
Naderalvojoud B, Shah ND, Mutanga JN, Belov A, Staiger R, Chen JH, Whitaker B, Hernandez-Boussard T. Trends in Influenza Vaccination Rates among a Medicaid Population from 2016 to 2021. Vaccines. 2023; 11(11):1712. https://doi.org/10.3390/vaccines11111712
Chicago/Turabian StyleNaderalvojoud, Behzad, Nilpa D. Shah, Jane N. Mutanga, Artur Belov, Rebecca Staiger, Jonathan H. Chen, Barbee Whitaker, and Tina Hernandez-Boussard. 2023. "Trends in Influenza Vaccination Rates among a Medicaid Population from 2016 to 2021" Vaccines 11, no. 11: 1712. https://doi.org/10.3390/vaccines11111712
APA StyleNaderalvojoud, B., Shah, N. D., Mutanga, J. N., Belov, A., Staiger, R., Chen, J. H., Whitaker, B., & Hernandez-Boussard, T. (2023). Trends in Influenza Vaccination Rates among a Medicaid Population from 2016 to 2021. Vaccines, 11(11), 1712. https://doi.org/10.3390/vaccines11111712





