Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®)
Abstract
:1. Introduction
2. Materials and Methods
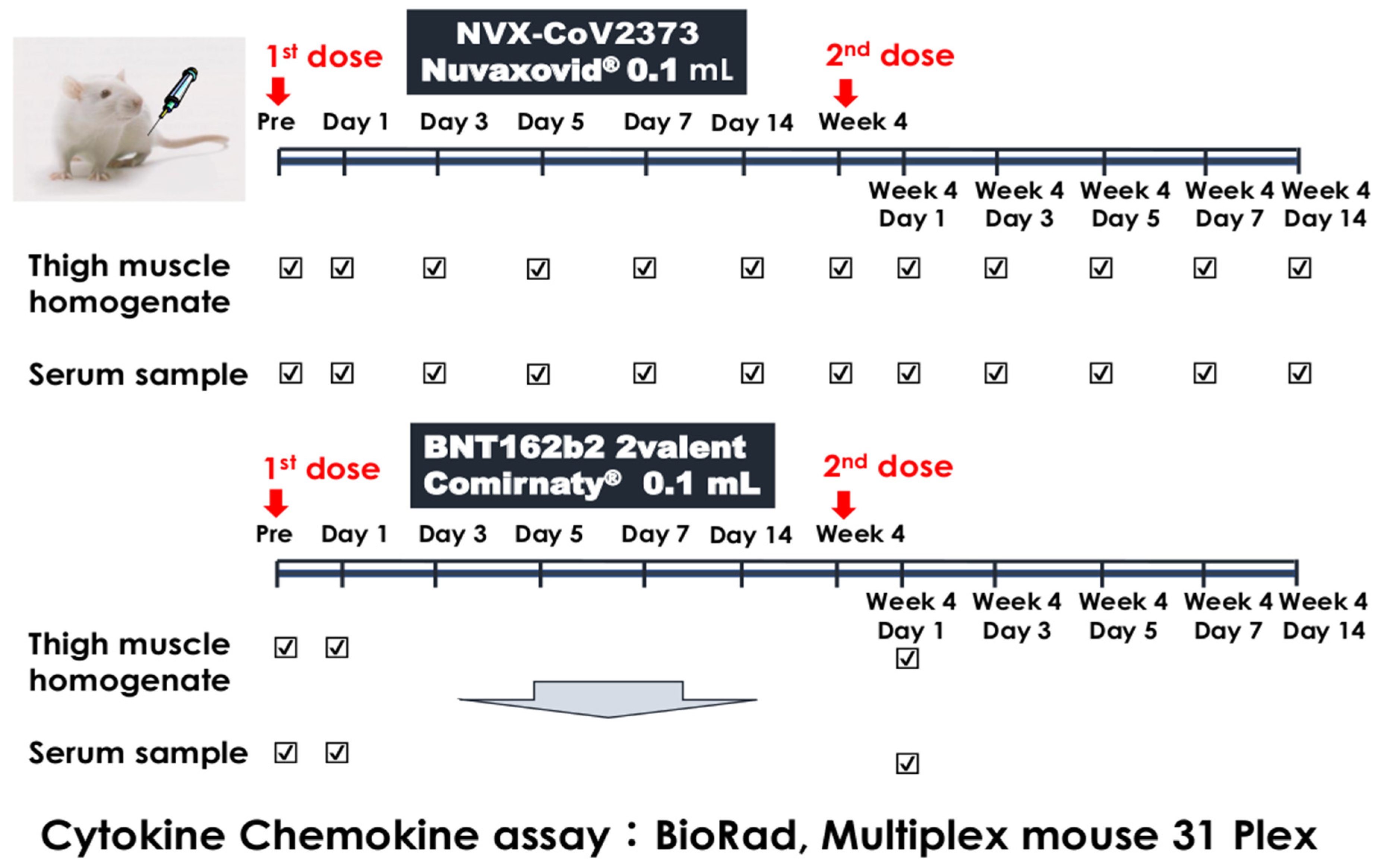
2.1. Experimental Protocol
2.2. Cytokine and Chemokine Assay
2.3. IgG EIA Antibody against SARS-CoV-2 Spike Protein
2.4. Statistical Analysis
3. Results
3.1. Cytokine Production Profiles following Inoculation with NVX-CoV2373
3.2. Chemokine Production Profiles following Inoculation with NVX-CoV2373
3.3. Comparison of Cytokines following NVX-CoV2373 and Bivalent BNT162b2
3.4. Comparison of Chemokines following NVX-CoV2373 and Bivalent BNT162b2
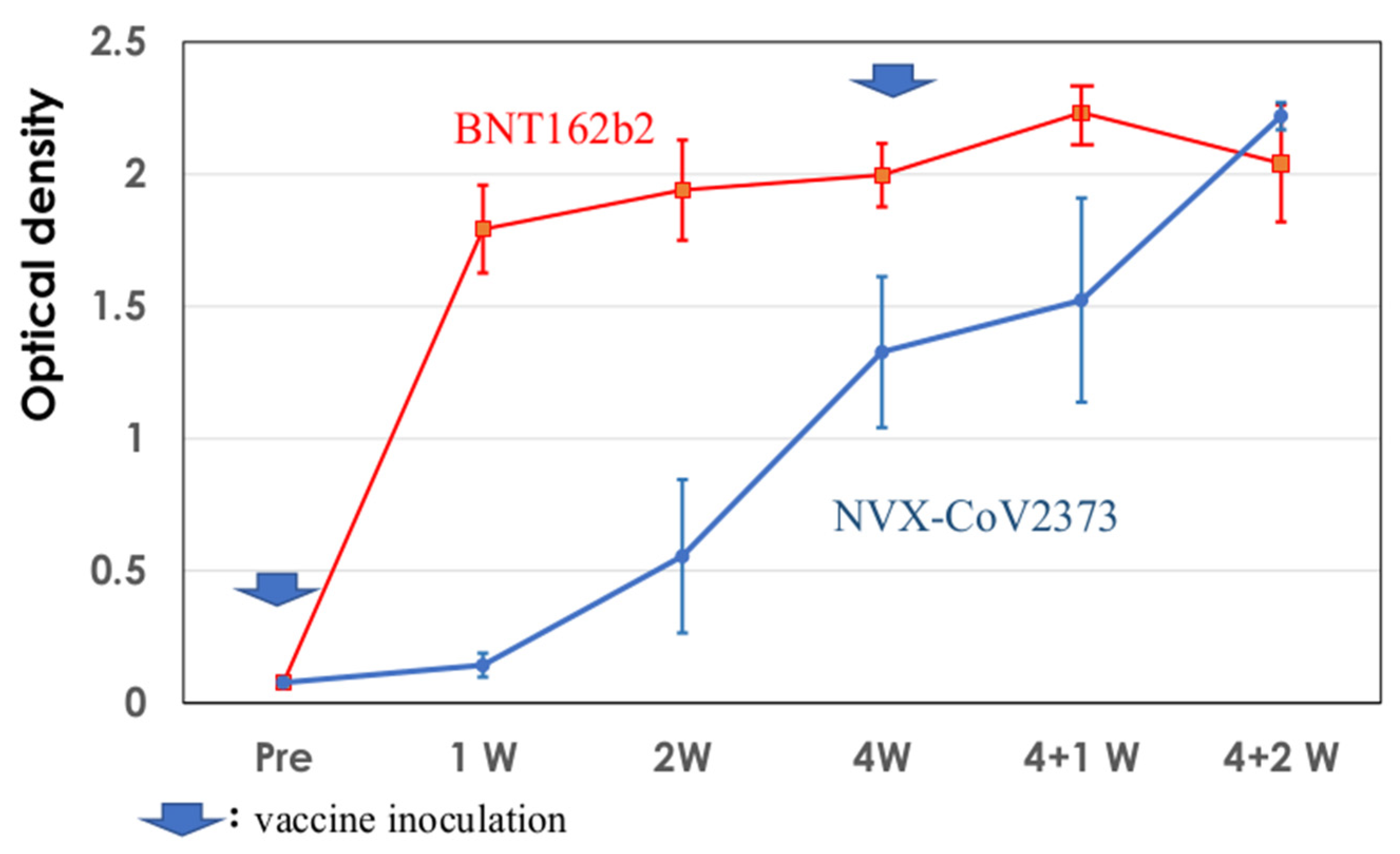
3.5. Development of IgG EIA Antibodies against SARS-CoV2 Spike Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Corey, L.; Mascola, J.R.; Fauci, A.S.; Collins, F.C. A strategic approach to COVID-19 vaccine R&D. Science 2020, 368, 948–950. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Nakayama, T.; Sawada, A.; Ito, T. Comparison of cytokine production in mice inoculated with messenger RNA vaccines BNT162b2 and mRNA-1273. Microbiol. Immunol. 2023, 67, 120–128. [Google Scholar] [CrossRef]
- Yoneyama, M.; Fujita, T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 2009, 20, 4–22. [Google Scholar] [CrossRef]
- Yu, M.; Levine, S.J. Toll-like receptor 3, RIG-I-like receptors and the NLRP3 inflammasome: Key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Fact. Rev. 2011, 22, 63–72. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Health, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Tang, P.; Gong, W.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef]
- Graham, G.J. D6 and the atypical chemokine receptor family: Novel regulators of immune and inflammatory processes. Eur. J. Immunol. 2009, 39, 342–351. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine networking of innate immunity cells: A potential target of therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef]
- Pollard, A.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Nakayama, T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine 2016, 34, 5815–5818. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Miyata, A.; Kumagai, T.; Maehara, K.; Suzuki, E.; Nagai, T.; Ozaki, T.; Nishimura, N.; Okada, K.; Kawashima, H.; et al. Production of inflammatory cytokines in response to diphtheria-pertussis-tetanus (DPT), haemophilus influenzae type b (Hib), and 7-valent pneumococcal (PCV7) vaccines. Hum. Vaccine Immunother. 2014, 10, 677–685. [Google Scholar] [CrossRef]
- Nakayama, T.; Kashiwagi, Y.; Kawashima, H. Long-term regulation of local cytokine production following immunization in mice. Microbiol. Immunol. 2018, 62, 124–131. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef]
- Stöver, A.G.; Correia, J.D.S.; Evans, J.T.; Cluff, C.W.; Elliott, M.W.; Jeffery, E.W.; Johnson, D.A.; Lacy, M.J.; Baldridge, J.R.; Probst, P.; et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J. Biol. Chem. 2004, 279, 4440–4449. [Google Scholar] [CrossRef]
- Chlibek, R.; Bayas, J.M.; Collins, H.; de la Pinta, M.L.R.; Ledent, E.; Mols, J.F.; Heineman, T.C. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J. Infect. Dis. 2013, 208, 1953–1961. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Nakayama, T.; Sawada, A.; Ito, T. Increased production of inflammatory cytokines after inoculation with recombinant zoster vaccine in mice. Vaccines 2022, 10, 1339. [Google Scholar] [CrossRef]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef]
- Masuda, T.; Murakami, K.; Sugiura, K.; Sakui, S.; Schuring, R.P.; Mori, M. Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: Interim report of a phase I/II randomized controlled trial. Vaccine 2022, 40, 3380–3388. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar]
- Harrer, C.; Otto, F.; Radlberger, R.F.; Moster, T.; Pilz, G.; Wipfler, P.; Harrer, A. The CXCL13/CXCR5 immune axis in health and disease—Implications for intrathecal B cell activities in neuroinflammation. Cells 2022, 11, 2649. [Google Scholar] [CrossRef]
- Lee, K.M.C.; Jarnicki, A.; Achuthan, A.; Fleetwood, A.J.; Anderson, G.P.; Ellson, C.; Feeney, M.; Modis, L.K.; Smith, J.E.; Hamilton, J.A.; et al. CCL17 in inflammation and pain. J. Immunol. 2020, 205, 213–222. [Google Scholar] [CrossRef]
- Wisnewski, A.V.; Luna, J.C.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Cloney-Clark, S.; Zhou, H.; Smith, G.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Mukhopadhyay, L.; Yadav, P.D.; Gupta, N.; Mohandas, S.; Patil, D.Y.; Shete-Aich, A.; Panda, S.; Bhargava, B. Comparison of the immunogenicity & protective efficacy of various SARS-CoV-2 vaccine candidates in non-human primates. Indian J. Med. Res. 2021, 153, 93–114. [Google Scholar] [CrossRef]
- Reimer, J.M.; Karlsson, K.H.; Lövgren-Bengtsson, K.; Magnusson, S.E.; Fuentes, A.; Stertman, L. Matrix-MTM adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS ONE 2012, 7, e41451. [Google Scholar] [CrossRef]
- Pearse, M.J.; Drane, D. ISCOMATRIX® adjuvant for antigen delivery. Adv. Drug. Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef]

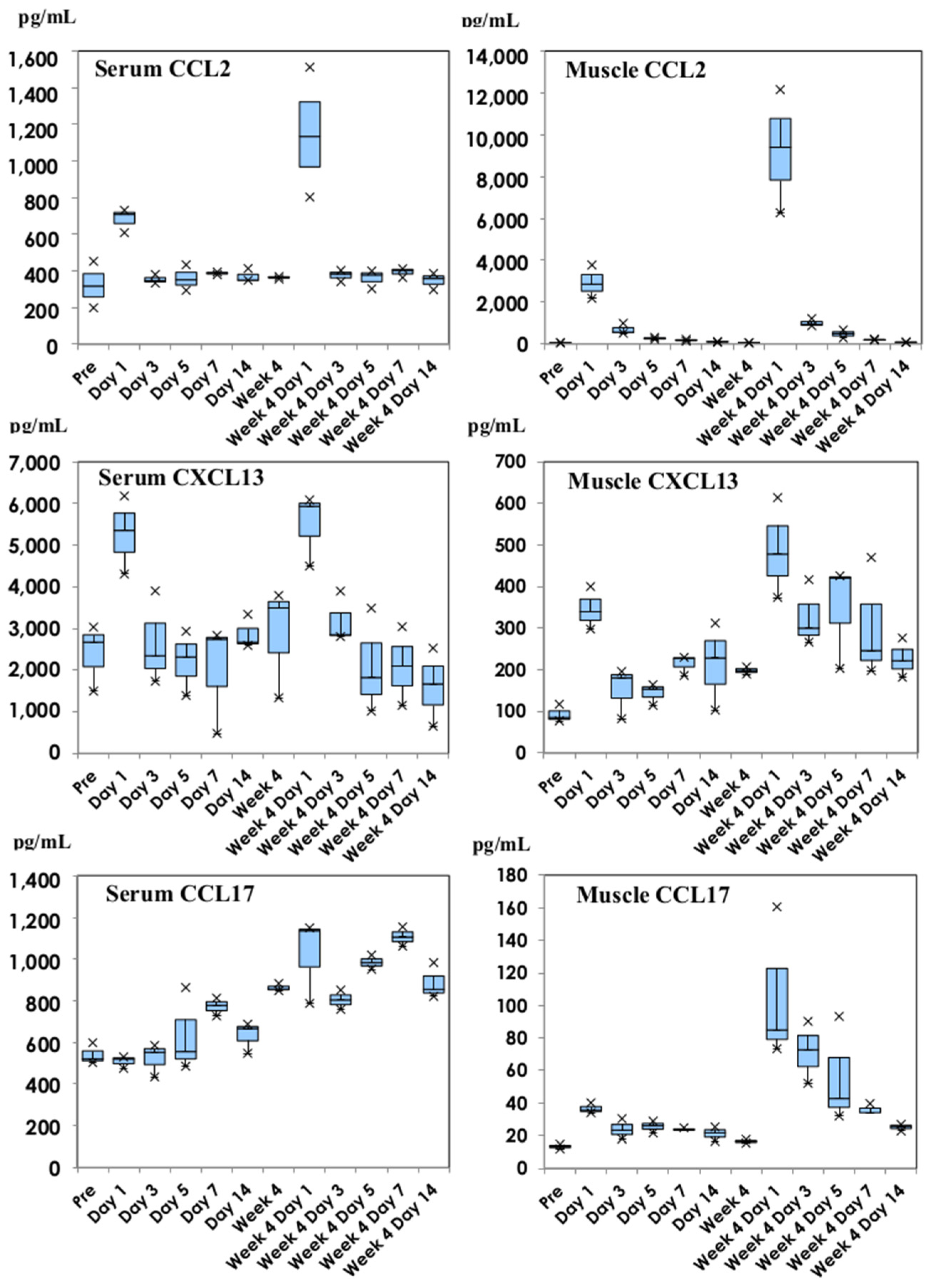
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05.
: Comirnaty®). * p < 0.05.
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05.
: Comirnaty®). * p < 0.05.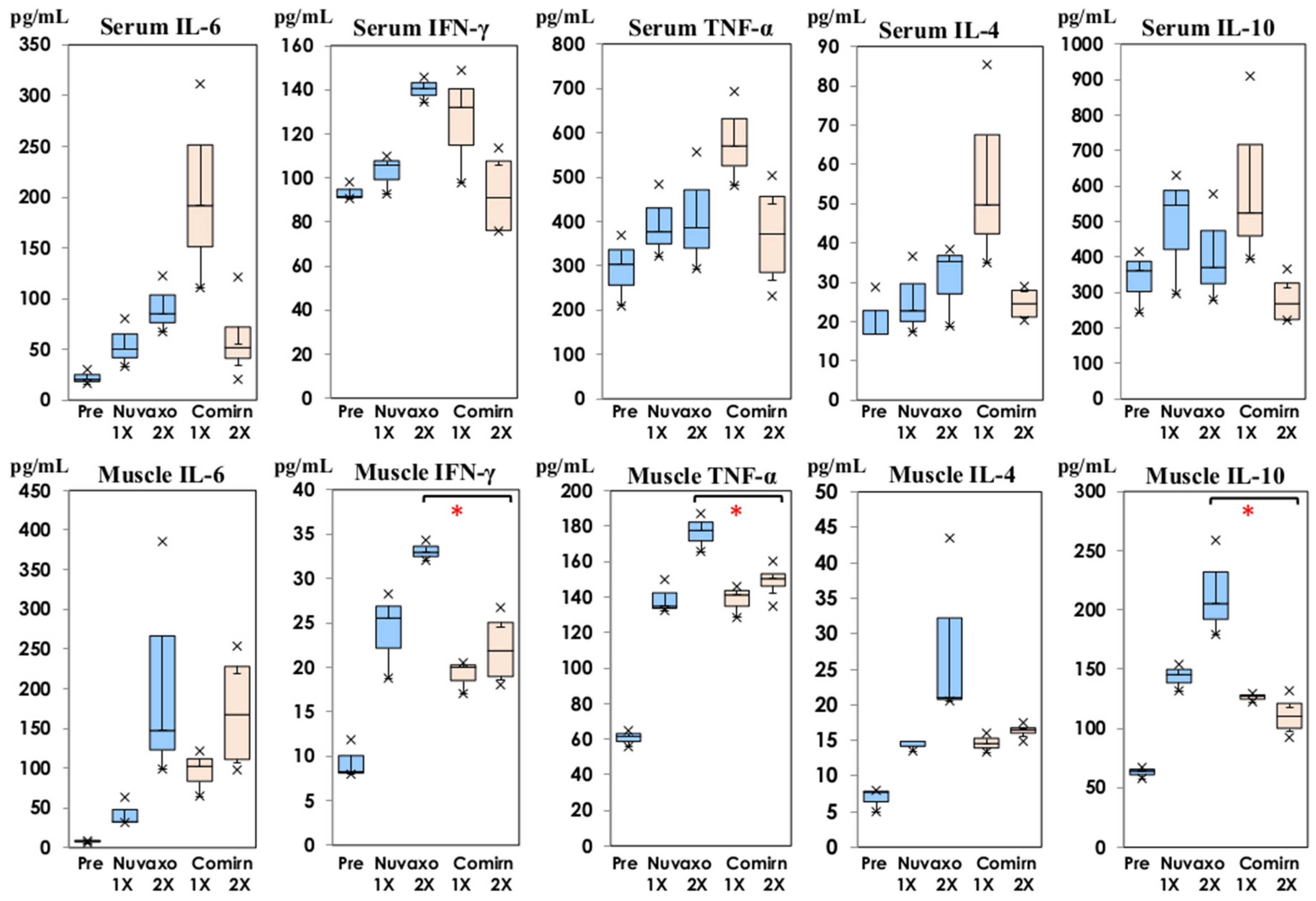
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). ** p < 0.01.
: Comirnaty®). ** p < 0.01.
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). ** p < 0.01.
: Comirnaty®). ** p < 0.01.
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05, ** p < 0.01.
: Comirnaty®). * p < 0.05, ** p < 0.01.
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05, ** p < 0.01.
: Comirnaty®). * p < 0.05, ** p < 0.01.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, T.; Ito, T.; Ishiyama, R.; Katayama, K. Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®). Vaccines 2023, 11, 1677. https://doi.org/10.3390/vaccines11111677
Nakayama T, Ito T, Ishiyama R, Katayama K. Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®). Vaccines. 2023; 11(11):1677. https://doi.org/10.3390/vaccines11111677
Chicago/Turabian StyleNakayama, Tetsuo, Takashi Ito, Ryoka Ishiyama, and Kazuhiko Katayama. 2023. "Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®)" Vaccines 11, no. 11: 1677. https://doi.org/10.3390/vaccines11111677
APA StyleNakayama, T., Ito, T., Ishiyama, R., & Katayama, K. (2023). Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®). Vaccines, 11(11), 1677. https://doi.org/10.3390/vaccines11111677






