Abstract
Introduction: Real-world safety studies can provide important evidence on the thromboembolic risk associated with COVID-19 vaccines, considering that millions of people have been already vaccinated against COVID-19. In this study, we aimed to estimate the incidence of thromboembolic events after COVID-19 vaccination and to compare the Oxford–AstraZeneca vaccine with other COVID-19 vaccines. Methods: We conducted a retrospective real-world safety study using data from two different data sources: the Italian Pharmacovigilance database (Rete Nazionale di Farmacovigilanza, RNF) and the Campania Region Health system (Sistema INFOrmativo saNità CampanIA, SINFONIA). From the start date of the COVID-19 vaccination campaign (27 December 2021) to 27 September 2022, information on COVID-19 vaccinations and thromboembolic events were extracted from the two databases. The reporting rate (RR) and its 95% confidence interval (95%CI) of thromboembolic events for 10,000 doses was calculated for each COVID-19 vaccine. Moreover, the odds of being vaccinated with the Oxford–AstraZeneca vaccine vs. the other COVID-19 vaccines in cases with thromboembolic events vs. controls without thromboembolic events were computed. Results: A total of 12,692,852 vaccine doses were administered in the Campania Region, of which 6,509,475 (51.28%) were in females and mostly related to the Pfizer-BioNtech vaccine (65.05%), followed by Moderna (24.31%), Oxford–AstraZeneca (9.71%), Janssen (0.91%), and Novavax (0.02%) vaccines. A total of 641 ICSRs with COVID-19 vaccines and vascular events were retrieved from the RNF for the Campania Region, of which 453 (70.67%) were in females. Most ICSRs reported the Pfizer-BioNtech vaccine (65.05%), followed by Oxford–AstraZeneca (9.71%), Moderna (24.31%), and Janssen (0.91%). A total of 2451 events were reported in the ICSRs (3.8 events for ICSRs), of which 292 were thromboembolic events. The higher RRs of thromboembolic events were found with the Oxford–AstraZeneca vaccine (RR: 4.62, 95%CI: 3.50–5.99) and Janssen vaccine (RR: 3.45, 95%CI: 0.94–8.82). Thromboembolic events were associated with a higher likelihood of exposure to the Oxford–AstraZeneca vaccine compared to Pfizer-BioNtech (OR: 6.06; 95%CI: 4.22–8.68) and Moderna vaccines (OR: 6.46; 95%CI: 4.00–10.80). Conclusion: We observed a higher reporting of thromboembolic events with viral-vector-based vaccines (Oxford–AstraZeneca and Janssen) and an increased likelihood of being exposed to the Oxford–AstraZeneca vaccine compared to the mRNA vaccines (Pfizer-BioNtech and Moderna) among thromboembolic cases.
1. Introduction
The rapid development of coronavirus disease 2019 (COVID-19) vaccines has helped change the course of the pandemic by reducing illness severity and hospital admissions [1]. As of January 2023, the European Medicines Agency (EMA) had granted authorization to seven COVID-19 vaccines [2]. Among these, two are mRNA vaccines: BNT162b2 mRNA (produced by Pfizer-BioNTech, approved on 21 December 2020) and mRNA-1273 (Moderna, approved on 6 January 2021). Additionally, two vaccines are based on adenovirus technology: ChAdOx1 nCoV-19 (Oxford–AstraZeneca, approved on 29 January 2021) and Ad.26.COV2.S (Janssen, approved on 11 March 2021); two of them are two recombinant-protein-based adjuvanted vaccines: NVX-CoV2373 (Novavax, 20 December 201) and Vidprevtyn (Sanofi, 10 November 2022); and one is an inactivated adjuvanted vaccine: VLA2001 (Valneva, 24 June 2022). Since their introduction, these vaccines have been associated with safety concerns [3]. Specifically, in March 2021, in response to spontaneous reports of thromboembolic events coupled with thrombocytopenia among individuals who received the Oxford–AstraZeneca vaccine, several European countries temporarily halted the administration of this vaccine [4]. Initially, these reports included 62 cases of cerebral venous sinus thrombosis (CVST) and 24 cases of splanchnic venous thrombosis (SVT) were reported with the Oxford–AstraZeneca vaccine in the European Union and United Kingdom (with 25,000,000 doses administered) [5]. In April 2021, thromboembolic events with thrombocytopenia were also reported with the Janssen vaccine [6]. The Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA confirmed a plausible causal relationship between rare events of thrombosis with thrombocytopenia and adenovirus-based vaccines [7,8]. Subsequently, in May 2021, the EMA published recommendations for the monitoring and prevention of thrombosis with the Oxford–AstraZeneca vaccine [9]; in November 2021, cases of CVST without thrombocytopenia were also observed with this vaccine leading the PRAC to amend its Summary of Product Characteristics to include this adverse event [10]. In the literature, two population-based studies showed an increased risk of thromboembolic events among people vaccinated with one dose of the Oxford–AstraZeneca vaccine compared to that observed in the general population [11,12]. Although far less reported, such events were also observed with mRNA vaccines [13]. A population-based studies conducted on over 2 million people vaccinated against COVID-19 found a risk of pulmonary embolism with the Pfizer-BioNtech vaccine and a risk of thrombocytopenia following the Pfizer-BioNtech and Oxford–AstraZeneca vaccinations [3]. In this context, real-world safety studies can provide important evidence on the safety of COVID-19 vaccines, considering that millions of people have been already vaccinated against COVID-19. In this study, we aimed to estimate the incidence of thromboembolic events after COVID-19 vaccination and to compare the Oxford–AstraZeneca vaccine with other COVID-19 vaccines using real-life data.
2. Methods
2.1. Study Design and Data Source
We conducted a retrospective real-world safety study using data from two different data sources: the Italian Pharmacovigilance database (Rete Nazionale di Farmacovigilanza, RNF) and the Campania Region Health system (Sistema INFOrmativo saNità CampanIA, SINFONIA). The RNF is the pharmacovigilance database managed by the Italian Medicine Agency (Agenzia Italiana del Farmaco, AIFA) containing all spontaneous reports of suspected adverse reactions to medicinal products or vaccines relevant to the national territory. The spontaneous reports were recorded according to general practitioners, physician evaluations, or any other healthcare professional. SINFONIA is the regional health information system designed to support the entire health service of the Campania Region (South of Italy) by increasing efficiency, containing costs, and enhancing the needs of all healthcare players (healthcare professionals, citizens, health structures, and institutions). SINFONIA contains socio-demographic and healthcare data of all Campania Region residents, including data on COVID-19 vaccinations.
2.2. Data Extraction
For the period from the start date of the COVID-19 vaccination campaign (27 December 2021) to 27 September 2022, information about COVID-19 vaccinations administered in the Campania Region, including patients’ characteristics (age and sex), type of vaccine, and vaccine dose, were extracted from SINFONIA in an anonymized and aggregated form according to our previous research protocol [14]. Briefly Machine learning (ML) analysis was conducted using a Python scripting model within the Spyder IDE (64-bit version). The analysis aimed to select, extract, and match individuals based on their vaccination status (vaccinated/negative or vaccinated/positive) and to perform forecasting analysis on contagiousness and coverage trends. The obtained data were further organized using Tableau Professional. In essence, machine learning algorithms leverage data to establish relationships between different data points and then export this information to another software for statistical analysis and graphical representation. Additionally, these algorithms create predictive models to forecast future trends and outcomes. These models essentially represent the actions the machine will take to achieve a specific result. This approach takes into account three primary elements: Event (positive or negative test), Category (type and time of subject—vaccinated or unvaccinated), and Time (t). The following formula is used for trend evaluation: Time series (Ts) = (Ep/En + Cv/Cu). During the same period, Individual Case Safety Reports (ICSRs) reporting at least one COVID-19 vaccine as suspected and one vascular event were retrieved from the RNF for the Campania Region. Vascular events were recognized using the System Organ Class (SOC) “Vascular disorders” of the Medical Dictionary for Regulatory Activities (MedDRA), version 25.1. From ICSRs, the information on patients’ characteristics (age and sex), thromboembolic events, and seriousness was available. In accordance with the International Council on Harmonization E2D guidelines, a case was serious if at least one adverse event was life-threatening, resulted in death, caused/prolonged hospitalization, was disabling, determined a congenital anomaly/birth defect, or was another medically important condition. On 27 December 2021, the following COVID-19 vaccines were administered in the Campania Region: Pfizer-BioNtech, Moderna, Oxford–AstraZeneca, Janssen, and Novavax vaccines. Therefore, data were retrieved only for those aforementioned vaccines. The adverse drug reaction severity classification has been made according to the FDA.
2.3. Descriptive and Statistical Analyses
Descriptive analyses were performed separately for the information reported in the two data sources and illustrated as numbers and percentages. The reporting rate (RR) and its 95% confidence interval (95%CI) of thromboembolic events for 10,000 doses was calculated for each COVID-19 vaccine by dividing the numbers of events with the number of doses administered. For the association between thromboembolic events and COVID-19 vaccinations, the odds of being vaccinated with the Oxford–AstraZeneca vaccine vs. the other COVID-19 vaccines in cases with thromboembolic events vs. controls without thromboembolic events were computed. The odds ratios (ORs) and their 95%CI were applied for all comparisons. A 5% significance level was considered for analyses that were performed with R (version 3.2.2, R Development Core Team).
2.4. Ethics
According to the local legislation, a retrospective pharmacovigilance study does not require ethical approval. For the use of retrospective SINFONIA data, this study was conducted in accordance with the Declaration of Helsinki 1975 and its later amendments. The research did not involve a clinical study, and all patients’ data were fully anonymized and were analyzed retrospectively. For this type of study, formal consent was not required according to the current national established by the Italian Medicines Agency, and according to the Italian Data Protection Authority, neither ethical committee approval nor informed consent were required for anonymized data, as confirmed and approved by the Ethical Committee of “Aziende Ospedaliere di Rilievo Nazionale e di Alta Specializzazione—A.Cardarelli/Santobono—Pausilipon” (Protocol Number 00000926 of 11 January 2022).
3. Results
3.1. Descriptive Results from SINFONIA
During the study period, a total of 12,692,852 vaccine doses were administered in the Campania Region, of which 6,509,475 (51.28%) were in females. Most doses were administered in patients aged 12–69 years (N = 10,535,109; 91.56%), with the age group of 50–59 years as the most representative (N = 2,232,236; 17.59%). Most doses were related to the Pfizer-BioNtech vaccine (65.05%), followed by Moderna (24.31%), Oxford–AstraZeneca (9.71%), Janssen (0.91%), and Novavax (0.02%) vaccines. All data from SINFONIA are displayed in Table 1.

Table 1.
Doses of COVID-19 vaccines administered in Campania Region (Italy) from 27 December 2020 to 27 September 2022 and described for gender, age group, number of doses, and type of vaccine.
3.2. Descriptive Results from RNF
During the study period, a total of 641 ICSRs with COVID-19 vaccines and vascular events were retrieved from the RNF for the Campania Region, of which 453 (70.67%) were females aged over 40 years old. Most ICSRs reported the Pfizer-BioNtech vaccine (65.05%), followed by Oxford–AstraZeneca (9.71%), Moderna (24.31%), and Janssen (0.91%). One case reported both Oxford–AstraZeneca and Moderna vaccines as suspected vaccines. No case of vascular events related to Novavax vaccine was found. The majority of ICSRs referred to the first dose of COVID-19 vaccines (N = 329; 51.32%), followed by second (N = 159; 24.81%) and third doses (N = 52; 8.11%). The information on doses was not available for 101 ICSRs (15.76%). Most ICSRs reported at least one event classified as serious (N = 261; 40.72%). Characteristics of ICSRs for each COVID-19 vaccine are reported in Table 2. A total of 2451 events were observed in the ICSRs (3.8 events for ICSRs), of which 292 were thromboembolic events. The most reported event was thrombosis (N = 17), followed by venous thrombosis (N = 10), deep venous thrombosis (N = 9), and D-dimer of fibrin increased (N = 9). Thromboembolic events for each COVID-19 vaccine are illustrated in Table 3, while all events are in Table S1.

Table 2.
Individual Case Safety Reports (ICSRs) of Campania Region reporting a COVID-19 vaccine as suspected and at least one vascular event for the period from 27 December 2020 to 27 September 2022.

Table 3.
Thromboembolic events for COVID-19 vaccines reported in Campania Region from 27 December 2020 to 27 September 2022.
3.3. Statistical Results
The highest RRs of thromboembolic events were found with the Oxford–AstraZeneca vaccine (RR: 4.62, 95%CI: 3.50–5.99) and Janssen vaccine (RR: 3.45, 95%CI: 0.94–8.82). All RRs are reported in Table 4. Thromboembolic events were associated with a higher likelihood of exposure to the Oxford–AstraZeneca vaccine compared to Pfizer-BioNtech (OR: 6.06; 95%CI: 4.22–8.68) and Moderna vaccines (OR: 6.46; 95%CI: 4.00–10.80). No difference was instead observed in the comparison with the Janssen vaccine (Figure 1).

Table 4.
Reporting rates (RRs) of thromboembolic events for 10.000 doses for each COVID-19 vaccine administered in Campania Region from 27 December 2020 to 27 September 2022.
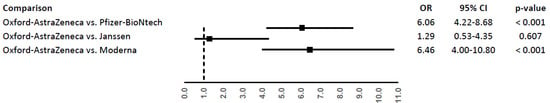
Figure 1.
Odds ratio (OR) of thromboembolic events for Oxford–AstraZeneca vaccine compared to Pfizer-BioNtech, Moderna, or Janssen vaccines.
4. Discussion
This study involving 12,692,852 vaccine doses administered in the Campania Region, Italy, showed increased RRs of thromboembolic events with the viral-vector-based vaccines (Oxford–AstraZeneca and Janssen). Moreover, an increased likelihood of being exposed to the Oxford–AstraZeneca vaccine compared to Pfizer-BioNtech and Moderna vaccines among thromboembolic cases was found. For instance, the ORs were 506- and 546-fold for thromboembolic events, respectively. These findings are consistent with the thromboembolic risk observed in clinical studies and issued by EMA [7,9,10,15].
In the literature, clinical studies on thromboembolic events and COVID-19 vaccinations are limited and with controversial results according to different studied population. A study conducted on VAERS data found no significantly increased risk after mRNA vaccines [16], and a Danish retrospective cohort also showed no statistically significant association between the onset of thromboembolic or thrombocytopenic events and the Pfizer-BioNtech vaccine [16]. Moreover, a real-world evidence-based study conducted on patients in the Mayo Clinic Health System observed that CVST is a rare event not significantly associated with COVID-19 vaccines [17]. One international network cohort study compared the thrombotic risk between COVID-19 vaccines. Similarly to our findings, this study showed an increased risk of thrombocytopenia when the Oxford–AstraZeneca vaccine was compared to Pfizer-BioNtech vaccine (pooled calibrated incidence rate ratio 1.33; 95%CI: 1.18–1.50) [18]. A Danish and Norwegian cohort study on 281,264 recipients aged 18–65 years found an increased standardized incidence rate of venous thromboembolism (1.97; 95%CI: 1.50–2.54) and thrombocytopenia (3.02; 95%CI: 1.76–4.83) within 28 days of vaccination [11]. Rates of venous thromboembolism were largely driven by events of CVST. Further, this study did not observe any increased rates of arterial thromboembolism [11]. A nested Scottish case–control study found increased rates of idiopathic thrombocytopenic purpura (ITP), arterial thromboembolism, and hemorrhagic events in 1.7 million recipients of a first dose of the Oxford–AstraZeneca vaccine [12]. A similar risk of ITP was also found in a post hoc self-controlled case series analysis [13]. Another similar study showed an increased risk of hospital admissions or deaths due to events of thromboembolism among vaccine recipients. Specifically, the first dose of the Oxford–AstraZeneca vaccine was associated with thrombocytopenia, venous thromboembolism, and CVST, while the first dose of the Pfizer-BioNtech vaccine was associated with arterial thromboembolism, ischaemic stroke, and CVST [19]. In contrast, a cohort study did not find an overall association between Pfizer-BioNtech and arterial thromboembolism, but it was found in a subgroup analysis of recipients aged 50–69 years [3]. Another cohort study also showed an increased risk of pulmonary embolism among recipients of a first dose of the Pfizer-BioNtech vaccine [20]. Moreover, risks of pulmonary embolism and thrombocytopenia were found to be increased in recipients of the Oxford–AstraZeneca vaccine, and the risk of immune thrombocytopenia was found to be high for both Oxford–AstraZeneca and Pfizer-BioNtech vaccines [20]. In our study, due to the limited number of each specific event, we could only perform an analysis for the aggregated number of thromboembolic events. However, in terms of reporting, we observed that venous thromboembolism events were more reported than arterial thromboembolism events and reported after the first dose of vaccination, also in accordance with results from a systematic review [14].
The connection between the potential risk of hemorrhagic stroke and BNT162b2 remains unclear. This uncertainty could arise from the interaction between the spike protein of SARS-CoV-2 and platelets, potentially elevating the risk of thromboembolic events in SARS-CoV-2-infected patients, thereby contributing to significant bleeding incidents. The spike protein, targeted by both mRNA- and vector-based vaccines, might lead to a syndrome resembling thrombosis and thrombocytopenia in vaccine recipients, similar to heparin-induced thrombocytopenia in patients [21]. This parallels the current understanding of the observed risk of thrombocytopenic thrombosis associated with Vaxzevria [19]. Despite this, the mechanisms responsible for a potential link between COVID-19 vaccines and thromboembolic events are currently under investigation. Following the initial alerts regarding the Oxford–AstraZeneca vaccine, a new immune disorder known as vaccine-induced immune thrombotic thrombocytopenia emerged is being described in the literature for this vaccine [21,22]. This new event occurs as an atypical thrombosis associated with thrombocytopenia, including CVST, from 5 to 15 days after the vaccination, and it might be mediated by the cross-reactivity between antibodies generated after vaccination and platelet factor 4 (PF4) [14]. However, there is also evidence that does not support this hypothesis [23]. Some authors have also proposed that the inflammatory response after vaccination may increase the clearance mediated by macrophages and/or reduce the platelet production, thus inducing thrombocytopenia [13]. These mechanisms have been previously postulated for the ITP following viral infections [24], as well as vaccination against other viruses (such as measles-mumps-rubella and varicella-zoster) [25,26]. Another mechanism specific for viral-vector-based vaccines involves the adenovirus carrier deliveries of DNA encoding the Spike (S) protein to the pulmonary megakaryocytes via the coxsackie-adenovirus receptor (CAR). This leads to megakaryocyte activation, biogenesis of activated platelets, and release of thromboxane A2 (TxA2) and PF4 that further activates platelets and their traversal through the cerebral vein sinuses, leading to thromboinflammation, CVST, and thromboembolism in other blood vessels [27,28,29]. Moreover, vaccines containing the adenovirus as vector may bind the PF4, leading to the formation of an immunogenic complex (like the heparin–PF4 complex), which can cause platelet activation and thrombosis [30]. Generally, females are associated with a more pronounced platelet activation, and hence, a higher risk of thromboembolic events after vaccination [31]. Indeed, a higher reporting of thromboembolic events in females (71%) was observed.
To the best of our knowledge, the present study is the first conducted on BigData from a unique Italian Region (Campania) according to ML and AI algorithms [14]. The strength is the collection of aggregated data for more than 12,000,000 people vaccinated for COVID-19 using the large regional SINFONIA database that, as previously observed for other studies, represented the strength of our current research protocols [14,32]. Nonetheless, one of the significant limitations is the use of safety data from the spontaneous reporting system (the RNF), which is characterized by underreporting and poor quality of information. The underreporting could limit the observation of the real number of thromboembolic events that occurred in our regional territory. However, considering that a lower number of events may be identified, we may have underestimated rates of thrombotic adverse events. The poor quality of information limits our analysis from considering risk factors for thromboembolic events as well as the time between the vaccination and the onset of thromboembolic events. Furthermore, we could only use aggregated data from the SINFONIA database, therefore no information on vaccine doses for each COVID-19 vaccine was retrievable, and the analyses were limited on the total number of vaccine doses administered. Finally, an important limitation is that events retrieved from pharmacovigilance cases are not surely related to the vaccine but simply reported as a clinically significant event after vaccination. This latter concept is important to be highlighted in the pharmacovigilance field, and in fact, the spontaneous reporting is driven by the suspect and not even the certainty of an event–vaccine association.
5. Conclusions
In conclusion, we found a higher reporting of reported thromboembolic events with viral-vector-based vaccines (Oxford–AstraZeneca and Janssen) and an increased likelihood of being exposed to the Oxford–AstraZeneca vaccine compared to the mRNA vaccines (Pfizer-BioNtech and Moderna) among thromboembolic cases. COVID-19 vaccination remains the most effective prevention strategy to fight this pandemic, and any safety concern should be weighed against the advantages of being vaccinated. The continuous pharmacovigilance monitoring is fundamental to collect more information and help to improve the management of these rare but often severe thromboembolic events associated with COVID-19 vaccination. Further, the use of BigData, as SINFONIA in the Campania Region, associated with a machine learning algorithm for data extraction and analysis granted the possibility to have a deeper evaluation and potentially more helpful support in the evaluation of adverse events to drugs including vaccines [33].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines11101575/s1.
Author Contributions
Conceptualization, E.C. and A.P.; Methodology, A.M., R.R., L.S., M.B., P.D.M., A.C. and A.P.; Software, M.S., N.C. and G.M.F.; Validation, A.C. and A.P.; Formal analysis, A.M. and A.C.; Resources, F.F.B., U.T. and M.B.; Data curation, M.S.; Writing—review & editing, F.F.B., A.I. and A.P.; Visualization, F.F.B. and A.M.; Supervision, A.C. and A.P.; Project administration, A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Aziende Ospedaliere di Rilievo Nazionale e di Alta Specializzazione—A.Cardarelli/Santobono—Pausilipon” (Protocol Number 00000926 of 11 January 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from Campania Region and are available after permission.
Acknowledgments
This study has been conducted under the supervision of the Campania Regional Pharmacovigilance Center and the Campania Region Observatory for Infectious Disease.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stokel-Walker, C. What do we know about covid vaccines and preventing transmission? BMJ 2022, 376, o298. [Google Scholar] [CrossRef] [PubMed]
- EMA. COVID-19 Vaccines|European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 12 January 2023).
- Burn, E.; Roel, E.; Pistillo, A.; Fernández-Bertolín, S.; Aragón, M.; Raventós, B.; Reyes, C.; Verhamme, K.; Rijnbeek, P.; Li, X.; et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in Catalonia, Spain. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef] [PubMed]
- EMA. COVID-19 Safety Update Vaxzevria Vaccine—14 April 2021. 2021. Available online: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-14-april-2021_en.pdf (accessed on 12 January 2023).
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.A.; Shimabukuro, T.T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684. [Google Scholar] [CrossRef] [PubMed]
- EMA. Signal Assessment Report on Embolic and Thrombotic Events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [Recombinant])—Vaxzevria (Previously COVID-19 Vaccine AstraZeneca) (Other Viral Vaccines) EPITT no:19683. 2021. Available online: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant_en.pdf (accessed on 12 January 2023).
- EMA. COVID-19 Vaccine Janssen: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets|European Medicines Agency. 2020. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 12 January 2023).
- EMA. COVID-19 Vaccine Safety Update of 21 May 2021 VAXZEVRIA AstraZeneca AB. 2021. Available online: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-21-may-2021_en.pdf (accessed on 12 January 2023).
- EMA. COVID-19 Vaccine Safety Update of 11 November 2021 VAXZEVRIA AstraZeneca AB. 2021. Available online: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-11-november-2021_en.pdf (accessed on 12 January 2023).
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.-D.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Perrella, A.; Mucherino, S.; Guarino, I.; Nerilli, M.; Maraolo, A.E.; Capoluongo, N.; Coscioni, E.; Trama, U.; Menditto, E.; Orlando, V. Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study. Vaccines 2022, 10, 511. [Google Scholar] [CrossRef]
- Mani, A.; Ojha, V. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Ann. Vasc. Surg. 2022, 84, 12. [Google Scholar] [CrossRef]
- Lai, D.; Zhang, Y.D.; Lu, J. Venous Thromboembolism following Two Doses of COVID-19 mRNA Vaccines in the US Population, 2020–2022. Vaccines 2022, 10, 1317. [Google Scholar] [CrossRef]
- Hviid, A.; Hansen, J.V.; Thiesson, E.M.; Wohlfahrt, J. Association of AZD1222 and BNT162b2 COVID-19 Vaccination With Thromboembolic and Thrombocytopenic Events in Frontline Personnel: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Rincón-Hekking, J.; Awasthi, S.; Pandey, V.; Lenehan, P.; Venkatakrishnan, A.; Bade, S.; O’Horo, J.C.; Virk, A.; Swift, M.D.; et al. Cerebral Venous Sinus Thrombosis is not Significantly Linked to COVID-19 Vaccines or Non-COVID Vaccines in a Large Multi-State Health System. J. Stroke Cerebrovasc. Dis. 2021, 30, 105923. [Google Scholar] [CrossRef]
- Li, X.; Burn, E.; Duarte-Salles, T.; Yin, C.; Reich, C.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.; A Suchard, M.; Li, K.; et al. Comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with different covid-19 vaccines: International network cohort study from five European countries and the US. BMJ 2022, 379, e071594. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; E Warkentin, T.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef]
- Dotan, A.; Shoenfeld, Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J. Autoimmun. 2021, 121, 102663. [Google Scholar] [CrossRef]
- Perricone, C.; Ceccarelli, F.; Nesher, G.; Borella, E.; Odeh, Q.; Conti, F.; Shoenfeld, Y.; Valesini, G. Immune thrombocytopenic purpura (ITP) associated with vaccinations: A review of reported cases. Immunol. Res. 2014, 60, 226–235. [Google Scholar] [CrossRef]
- Cecinati, V.; Principi, N.; Brescia, L.; Giordano, P.; Esposito, S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum. Vaccines Immunother. 2013, 9, 1158–1162. [Google Scholar] [CrossRef]
- Stone, D.; Liu, Y.; Shayakhmetov, D.; Li, Z.-Y.; Ni, S.; Lieber, A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 2007, 81, 4866–4871. [Google Scholar] [CrossRef]
- Jin, Y.-Y.; Yu, X.-N.; Qu, Z.-Y.; Zhang, A.-A.; Xing, Y.-L.; Jiang, L.-X.; Shang, L.; Wang, Y.-C. Adenovirus type 3 induces platelet activation in vitro. Mol. Med. Rep. 2014, 9, 370–374. [Google Scholar] [CrossRef]
- Chander, C.K.; Ajay, G. Mechanism of Thrombosis with AstraZeneca and J & J Vaccines: Expert Opinion by Kate Chander Chiang & Ajay Gupta, MD|Leaders in Pharmaceutical Business Intelligence (LPBI) Group. 2021. Available online: https://pharmaceuticalintelligence.com/2021/04/14/mechanism-of-thrombosis-with-astrazeneca-and-j-j-vaccines-expert-opinion-by-kate-chander-chiang-ajay-gupta-md/ (accessed on 17 January 2023).
- Kim, S.-Y.; Kwon, W.-A.; Shin, S.-P.; Seo, H.K.; Lim, S.-J.; Jung, Y.-S.; Han, H.-K.; Jeong, K.-C.; Lee, S.-J. Electrostatic interaction of tumor-targeting adenoviruses with aminoclay acquires enhanced infectivity to tumor cells inside the bladder and has better cytotoxic activity. Drug Deliv. 2018, 25, 49–58. [Google Scholar] [CrossRef]
- Leng, X.-H.; Hong, S.Y.; Larrucea, S.; Zhang, W.; Li, T.-T.; López, J.A.; Bray, P.F. Platelets of female mice are intrinsically more sensitive to agonists than are platelets of males. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 376–381. [Google Scholar] [CrossRef]
- Perrella, A.; Bisogno, M.; D’Argenzio, A.; Trama, U.; Coscioni, E.; Orlando, V. Risk of SARS-CoV-2 Infection Breakthrough among the Non-Vaccinated and Vaccinated Population in Italy: A Real-World Evidence Study Based on Big Data. Healthcare 2022, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).