Mechanism of Innate Immune Response Induced by Albizia julibrissin Saponin Active Fraction Using C2C12 Myoblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Stimulation
2.3. Cell Viability Assay
2.4. ELISA
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
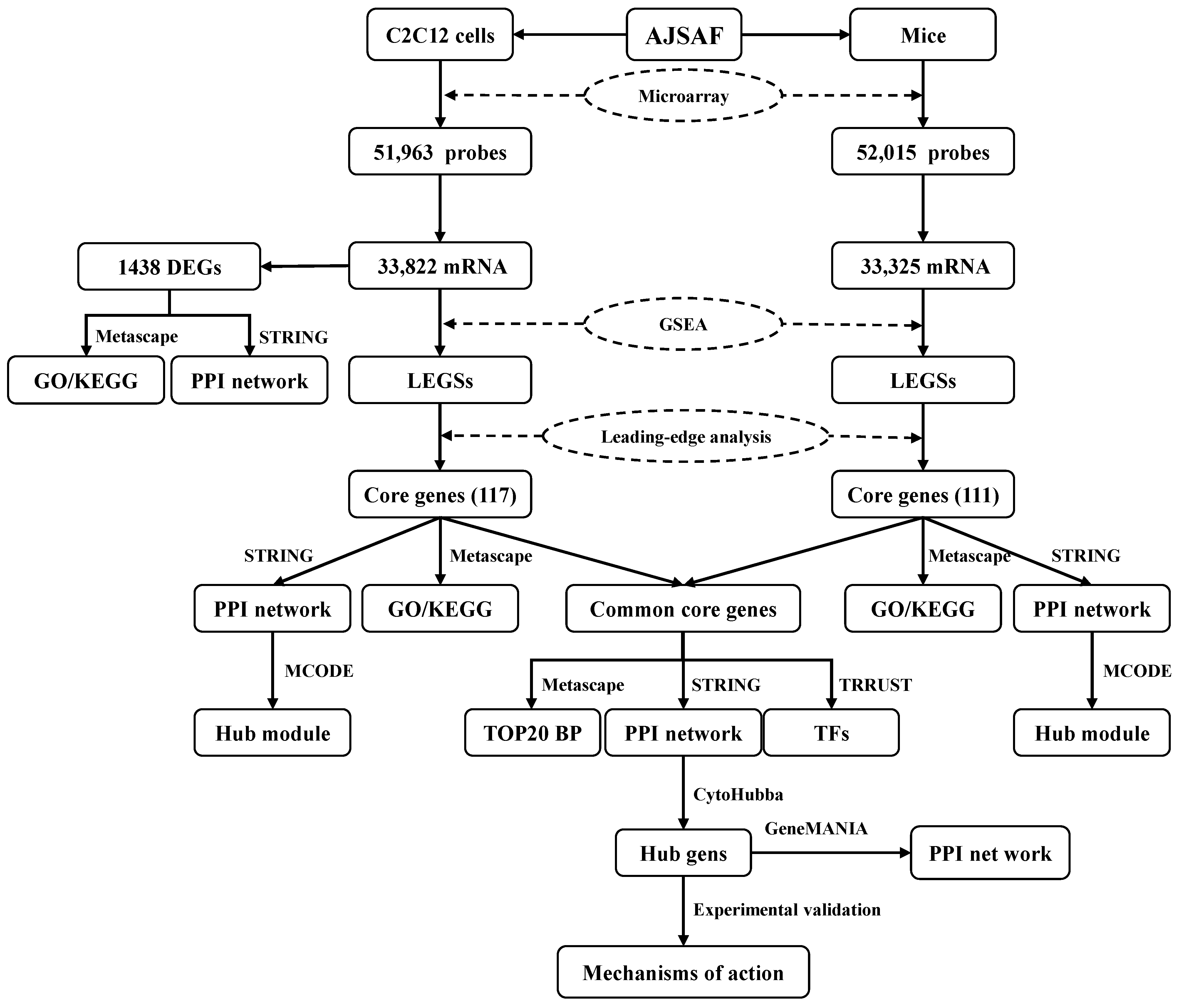
2.6. Microarray Analysis
2.7. Gene Set Enrichment Analysis (GSEA)
2.8. Relevance Analysis of Transcriptome In Vivo and In Vitro
2.9. cAMP, Free Ca2+ and ROS Detection
2.10. Western Blotting
2.11. Inhibition Assay
2.12. Statistical Analysis
3. Results
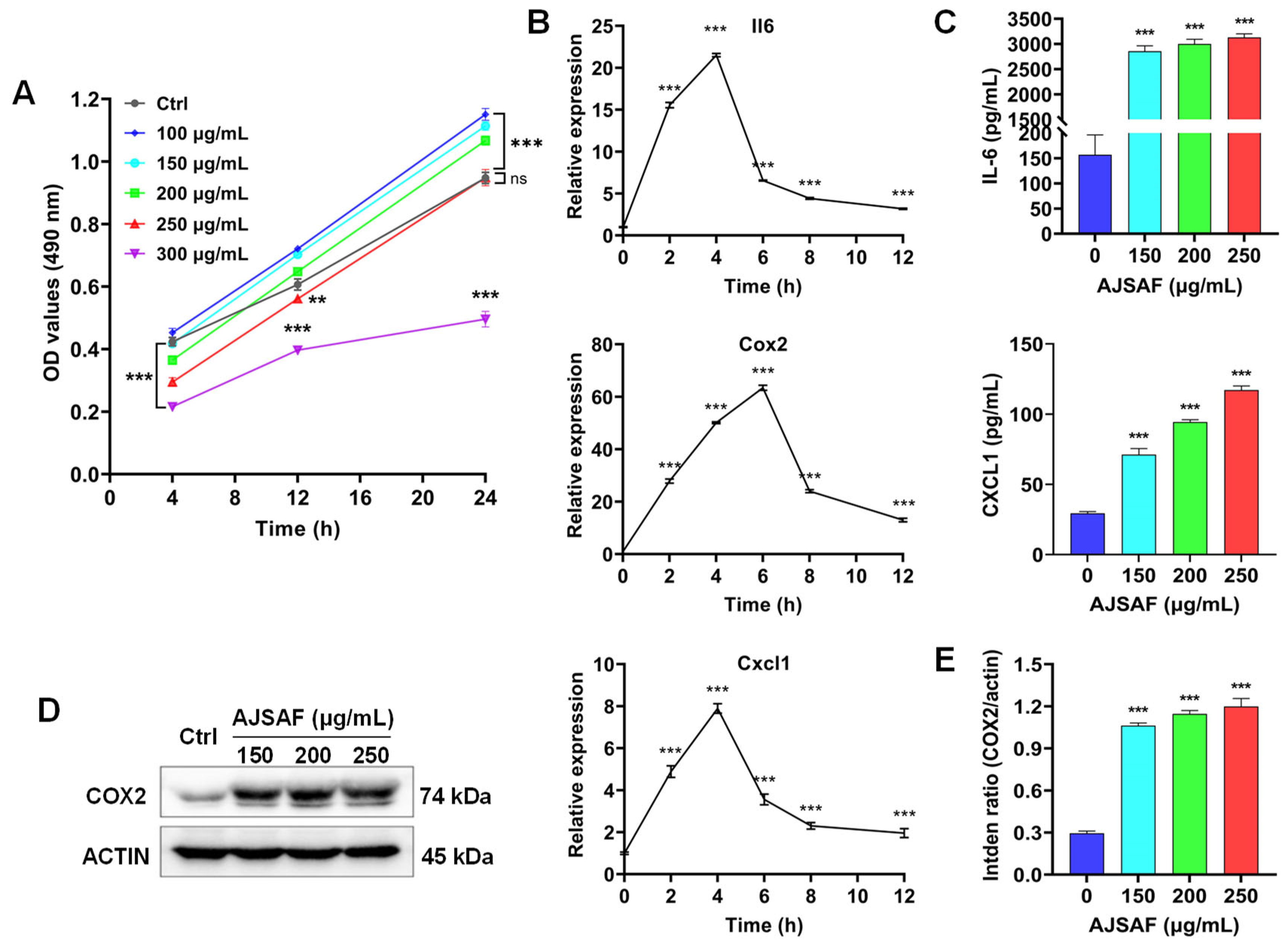
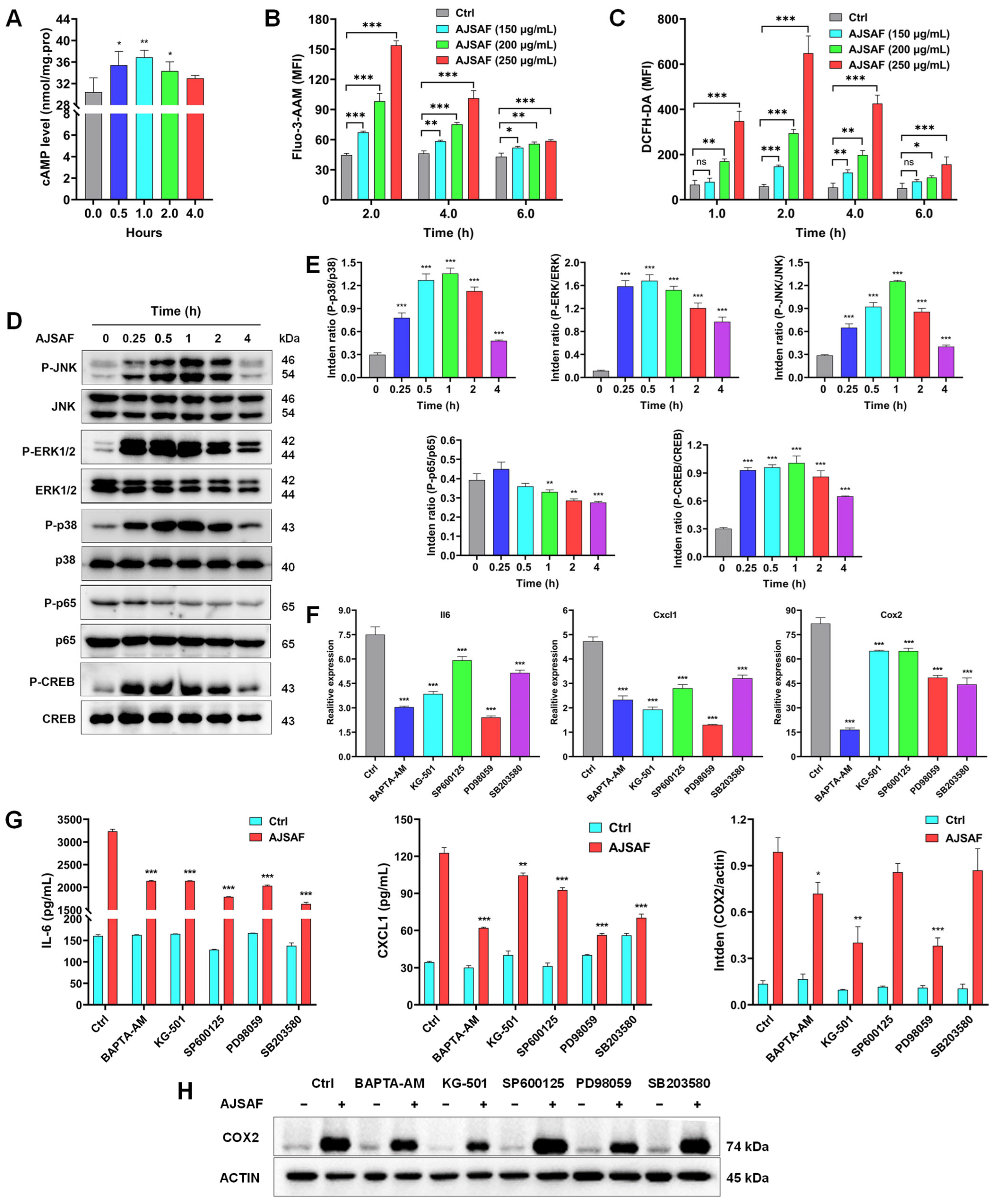
3.1. AJSAF Elicited a Temporary Cytotoxicity and Inflammation in C2C12 Cells
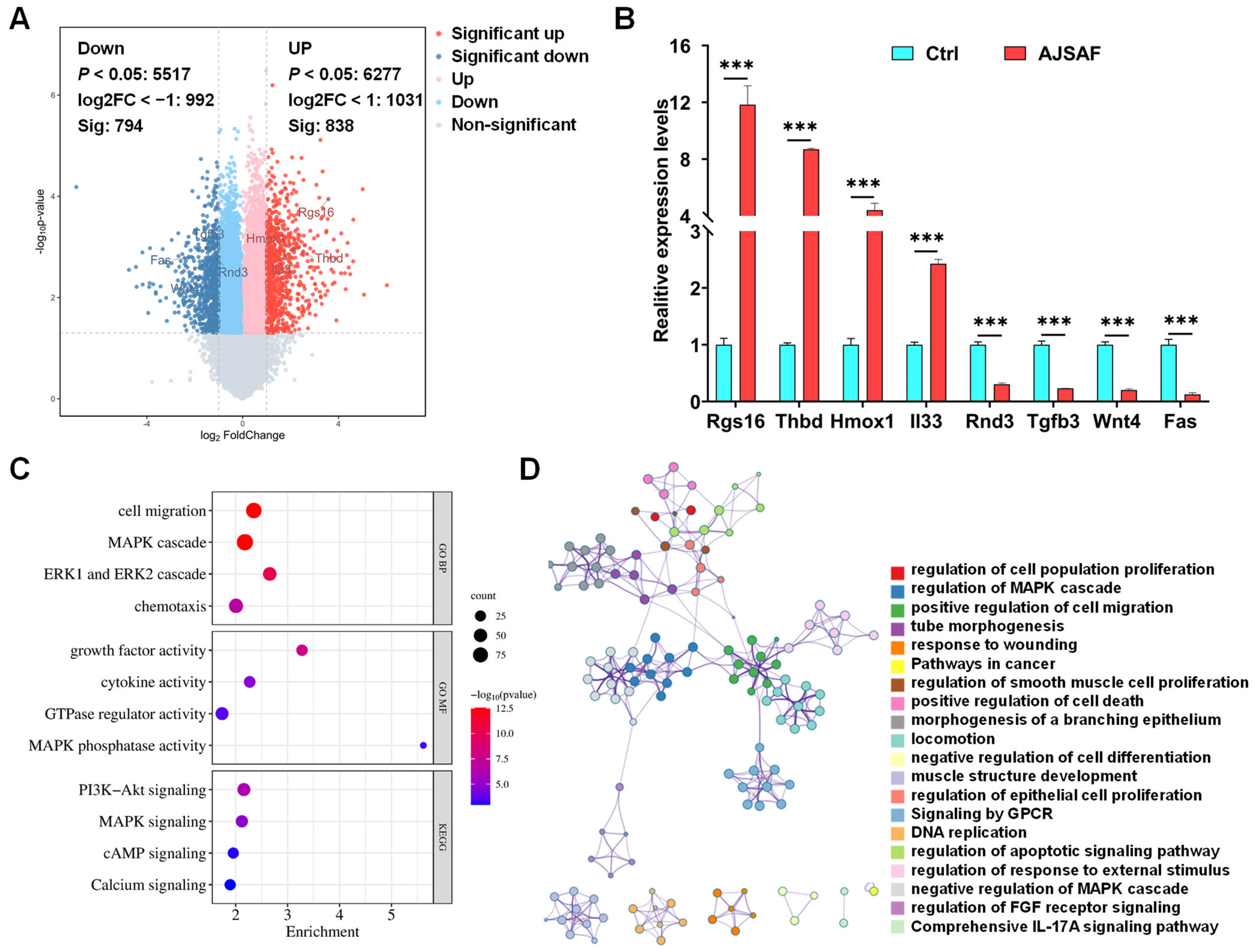
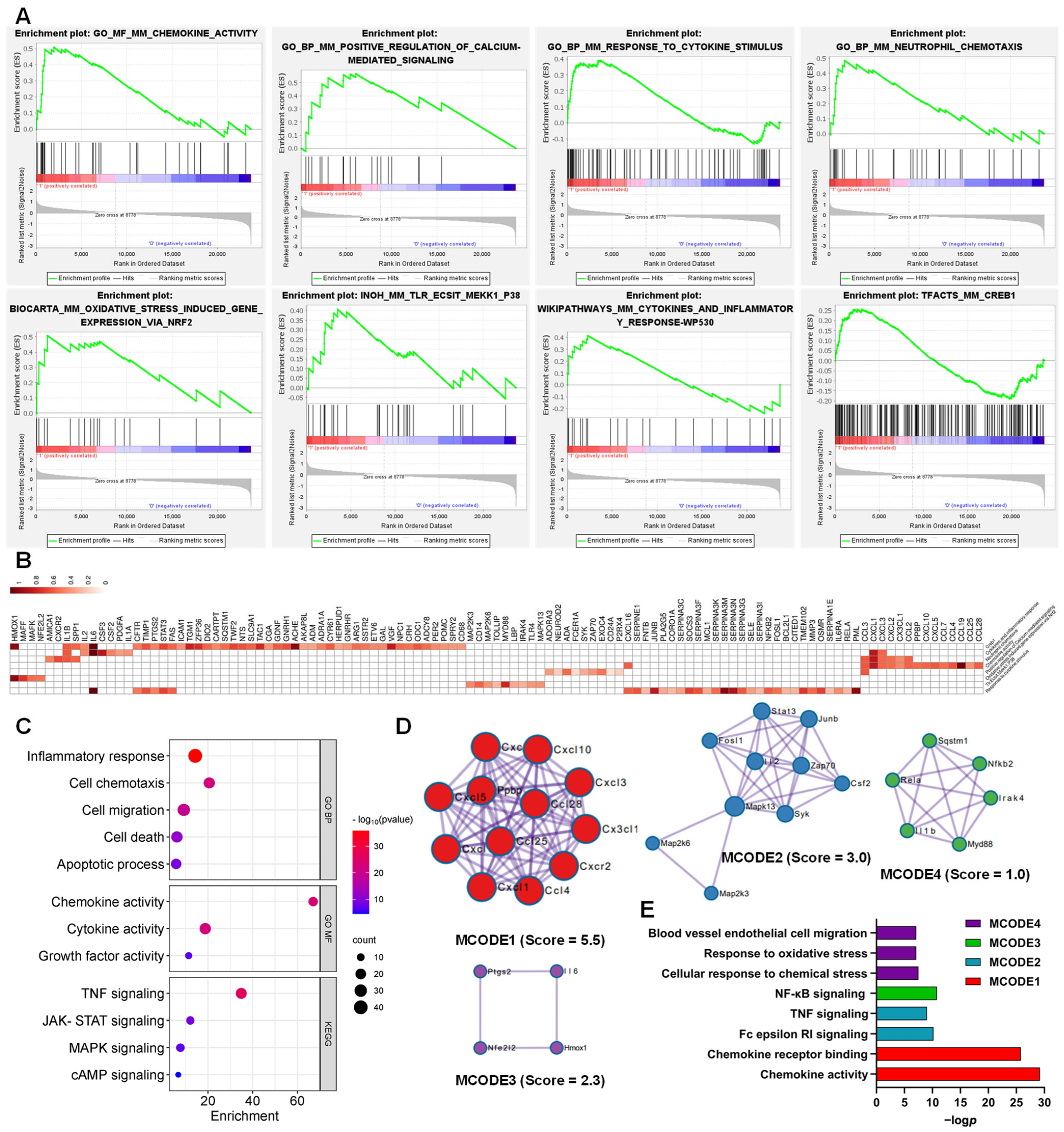
3.2. Functions and Pathways of AJSAF-Induced DEGs in C2C12 Cells
3.3. Function of the AJSAF-Induced Core Gens in C2C12 Cells
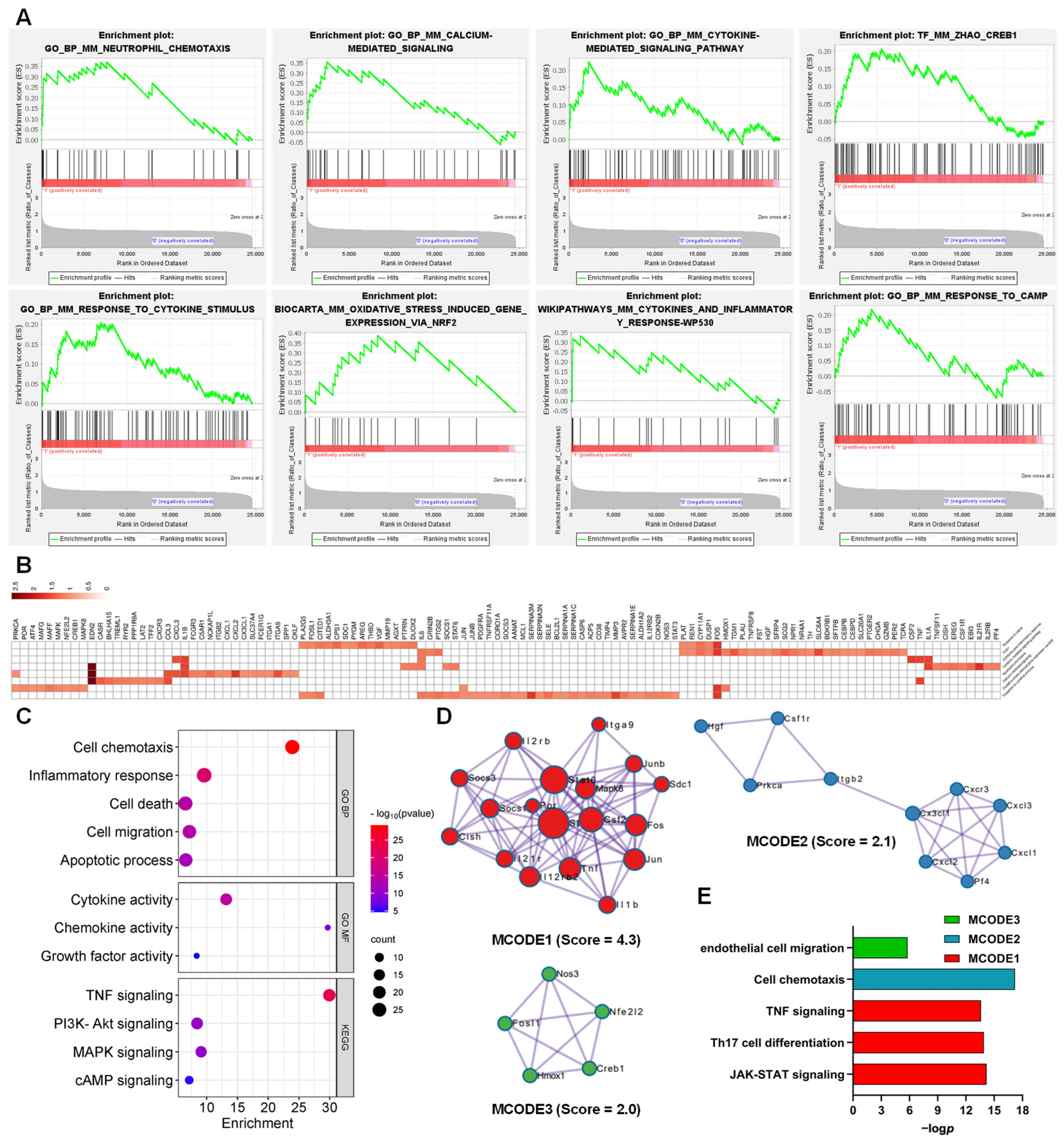
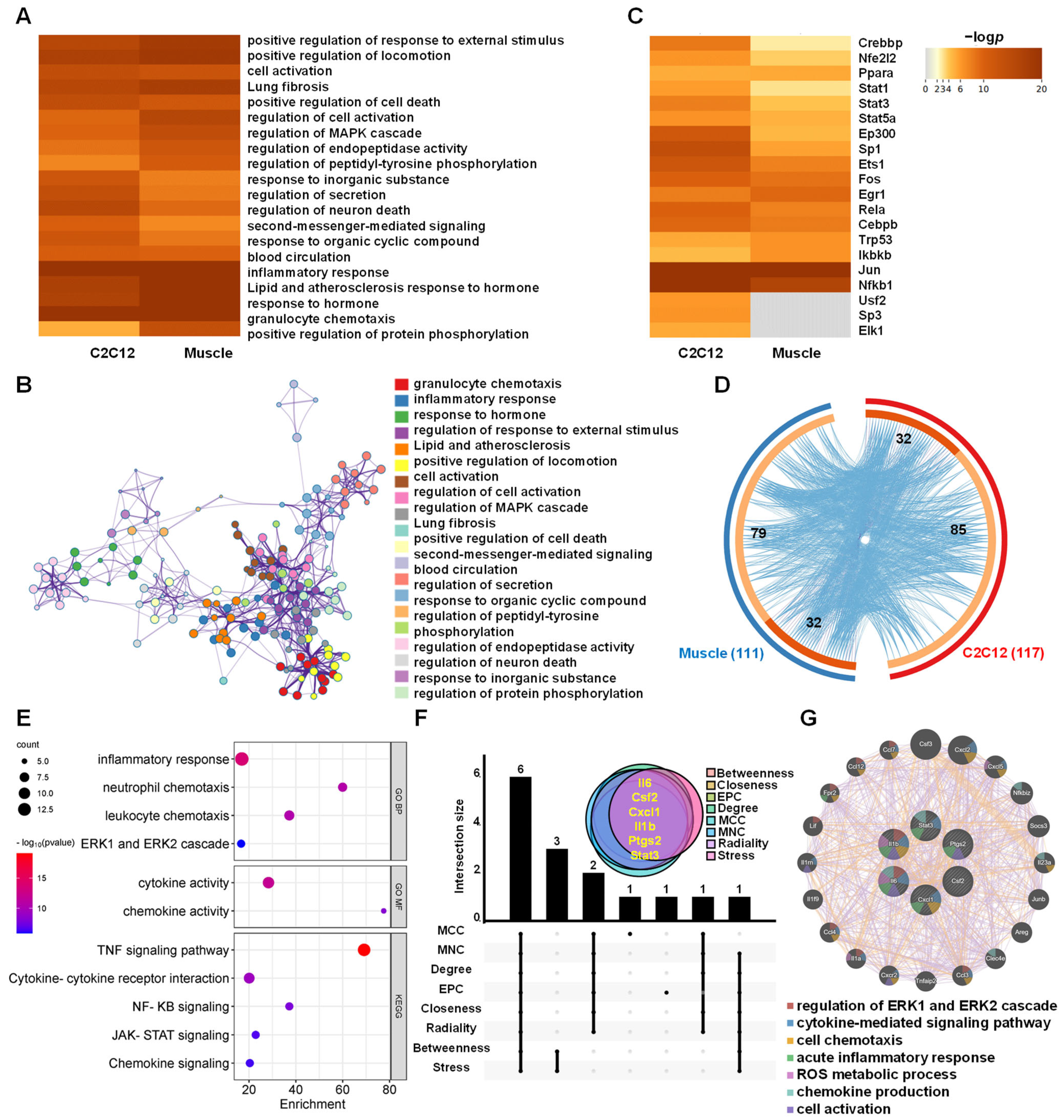
3.4. Functions of the AJSAF-Induced Core Gens in Mouse Quadricep Muscles
3.5. Functions and Hub Genes of AJSAF-Induced Common Core Genes In Vitro and In Vivo
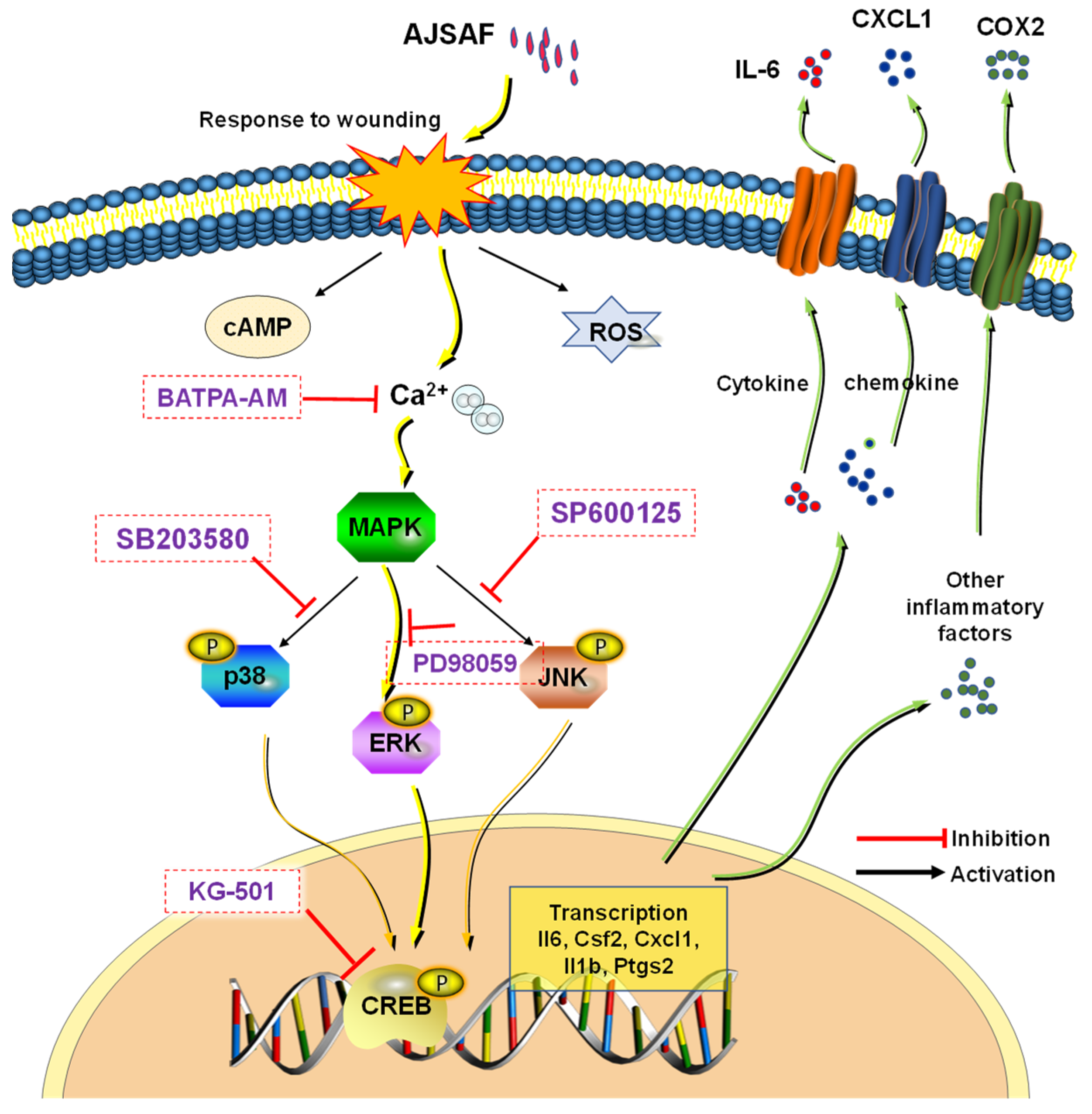
3.6. AJSAF Induced the Inflammation in C2C12 Cells through Ca2+–MAPK–CREB Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The continued advance of vaccine adjuvants—‘We can work it out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Du, J.; Jin, J.J.; Wang, J.J.; Sun, H.X. Mechanisms of mixed Th1/Th2 responses in mice induced by Albizia julibrissin saponin active fraction by in silico analysis. Vaccines 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Loré, K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin. Transl. Immunol. 2016, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Langlet, C.; Tamoutounour, S.; Henri, S.; Luche, H.; Ardouin, L.; Grégoire, C.; Malissen, B.; Guilliams, M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 2012, 188, 1751–1760. [Google Scholar] [CrossRef]
- Gornati, L.; Zanoni, I.; Granucci, F. Dendritic cells in the cross hair for the generation of tailored vaccines. Front. Immunol. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O.R.A. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Han, Z.Y.; He, Y.F.; Sun, H.X. Caspase-1-dependent pyroptosis mediates adjuvant activity of platycodin D as an adjuvant for intramuscular vaccines. Cells 2022, 11, 134. [Google Scholar] [CrossRef]
- Zhu, B.N.; He, T.Y.; Gao, X.Y.; Shi, M.H.; Sun, H.X. Evaluation and characteristics of immunological adjuvant activity of purified fraction of Albizia julibrissin saponins. Immunol. Investig. 2019, 48, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, H.X. Co-expression network analysis identifies innate immune signatures for Albizia julibrissin saponin active fraction-adjuvanted avian influenza vaccine. Int. Immunopharmacol. 2021, 93, 107417. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Liu, Z.Y.; Ye, Y.P.; Sun, H.X. Rapid annotation and structural characterization of saponins in the active fraction of Albizia julibrissin by HPLC coupled with quadrupole time-of-flight mass spectrometry based on accurate mass database. J. Sep. Sci. 2019, 42, 2922–2941. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.; Franceschini, A.; Wyder, S.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15558. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Han, H.; Cho, J.W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: An expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018, 46, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Du, J.; Chen, X.F.; Zhu, Y.L.; Sun, H.X. Activation of RAW264. 7 macrophages by active fraction of Albizia julibrissin saponin via Ca2+–ERK1/2–CREB–lncRNA pathways. Int. Immunopharmacol. 2019, 77, 105955. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Natural and synthetic saponins as vaccine adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils cascading their way to inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.B.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Gavala, M.L.; Pfeiffer, Z.A.; Bertics, P.J. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J. Leukoc. Biol. 2008, 84, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Palmai-Pallag, T.; Bachrati, C.Z. Inflammation-induced DNA damage and damage-induced inflammation: A vicious cycle. Microbes Infect. 2014, 16, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Pisetsky, D.S. The alarmin properties of DNA and DNA-associated nuclear proteins. Clin. Ther. 2016, 38, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Meng, X.; Ni, T.; Xiong, B.; Han, Z.; Zhu, Y.; Tu, J.; Sun, H. Mechanism of Innate Immune Response Induced by Albizia julibrissin Saponin Active Fraction Using C2C12 Myoblasts. Vaccines 2023, 11, 1576. https://doi.org/10.3390/vaccines11101576
Du J, Meng X, Ni T, Xiong B, Han Z, Zhu Y, Tu J, Sun H. Mechanism of Innate Immune Response Induced by Albizia julibrissin Saponin Active Fraction Using C2C12 Myoblasts. Vaccines. 2023; 11(10):1576. https://doi.org/10.3390/vaccines11101576
Chicago/Turabian StyleDu, Jing, Xiang Meng, Tiantian Ni, Beibei Xiong, Ziyi Han, Yongliang Zhu, Jue Tu, and Hongxiang Sun. 2023. "Mechanism of Innate Immune Response Induced by Albizia julibrissin Saponin Active Fraction Using C2C12 Myoblasts" Vaccines 11, no. 10: 1576. https://doi.org/10.3390/vaccines11101576
APA StyleDu, J., Meng, X., Ni, T., Xiong, B., Han, Z., Zhu, Y., Tu, J., & Sun, H. (2023). Mechanism of Innate Immune Response Induced by Albizia julibrissin Saponin Active Fraction Using C2C12 Myoblasts. Vaccines, 11(10), 1576. https://doi.org/10.3390/vaccines11101576





