Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sansone, E.; Tiraboschi, M.; Sala, E.; Albini, E.; Lombardo, M.; Castelli, F.; De Palma, G. Effectiveness of BNT162b2 vaccine against the B.1.1.7 variant of SARS-CoV-2 among healthcare workers in Brescia, Italy. J. Infect. 2021, 83, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Abufares, H.I.; Oyoun Alsoud, L.; Alqudah, M.A.Y.; Shara, M.; Soares, N.C.; Alzoubi, K.H.; El-Huneidi, W.; Bustanji, Y.; Soliman, S.S.M.; Semreen, M.H. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022, 23, 15415. [Google Scholar] [CrossRef]
- Noor, R. Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants. Viruses 2022, 14, 2541. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Boffetta, P.; Violante, F.; Durando, P.; De Palma, G.; Pira, E.; Vimercati, L.; Cristaudo, A.; Icardi, G.; Sala, E.; Coggiola, M.; et al. Determinants of SARS-CoV-2 infection in Italian healthcare workers: A multicenter study. Sci. Rep. 2021, 11, 5788. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Shimbashi, R.; Shiino, T.; Ainai, A.; Moriyama, S.; Arai, S.; Morino, S.; Takanashi, S.; Arashiro, T.; Suzuki, M.; Matsuzawa, Y.; et al. Specific COVID-19 risk behaviors and the preventive effect of personal protective equipment among healthcare workers in Japan. Glob. Health Med. 2023, 5, 5–14. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Lodi, V.; Feola, D.; De Palma, G.; Sansone, E.; Sala, E.; Janke, C.; Castelletti, N.; Porru, S.; Spiteri, G.; et al. Determinants of Anti-S Immune Response at 9 Months after COVID-19 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Viruses 2022, 14, 2657. [Google Scholar] [CrossRef]
- Department of Health and Social Care. GOV.UK. Joint Committee on Vaccination and Immunisation: Advice on Priority Groups for COVID-19 Vaccination, 30 December 2020. Available online: https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020 (accessed on 21 June 2023).
- Sansone, E.; Bonfanti, C.; Sala, E.; Renzetti, S.; Terlenghi, L.; Matteelli, A.; Tiraboschi, M.M.; Pedrazzi, T.; Lombardo, M.; Rossi, C.; et al. Immune Responses to SARS-CoV-2 Infection and Vaccine in a Big Italian COVID-19 Hospital: An 18-Month Follow-Up. Vaccines 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Turkkan, A.; Saglik, I.; Turan, C.; Sahin, A.; Akalin, H.; Ener, B.; Kara, A.; Celebi, S.; Sahin, E.; Hacimustafaoglu, M. Nine-month course of SARS-CoV-2 antibodies in individuals with COVID-19 infection. Ir. J. Med. Sci. 2022, 191, 2803–2811. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Agarwal, M.; Bear, J.; Burns, R.; Hu, X.; Korompoki, E.; Donohue, D.; Venzon, D.J.; et al. Sequential Analysis of Binding and Neutralizing Antibody in COVID-19 Convalescent Patients at 14 Months After SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 793953. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Visci, G.; Violante, F.S.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Larese Fillon, F.; Negro, C.; Janke, C.; Castelletti, N.; et al. Determinants of anti-S immune response at 6 months after COVID-19 vaccination in a multicentric European cohort of healthcare workers—ORCHESTRA project. Front. Immunol. 2022, 13, 986085. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, J.; Liu, Z.; Li, C.; Guo, Y.; Wang, Y.; Chen, K. Kinetics of severe acute respiratory syndrome coronavirus 2 infection antibody responses. Front. Immunol. 2022, 13, 864278. [Google Scholar] [CrossRef]
- ORCHESTRA Cohort. Publications—ORCHESTRA Cohort. Available online: https://orchestra-cohort.eu/publications/ (accessed on 21 June 2023).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. The New England journal of medicine. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. eBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.-D.; Jo, D.H.; Choe, K.-W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, L.; Chen, G. Is there a difference in the efficacy of COVID-19 vaccine in males and females?—A systematic review and meta-analysis. Hum. Vaccines Immunother. 2021, 17, 4741. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chiba, A.; Murayama, G.; Kuga, T.; Tamura, N.; Miyake, S. Sex, Age, and Ethnic Background Shape Adaptive Immune Responses Induced by the SARS-CoV-2 mRNA Vaccine. Front. Immunol. 2022, 13, 786586. [Google Scholar] [CrossRef]
- Weinberger, B.; Grubeck-Loebenstein, B. Vaccines for the elderly. Clinical microbiology and infection: The official publication of the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2012, 18 (Suppl. S5), 100–108. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Interim Analysis of COVID-19 Vaccine Effectiveness Against Severe Acute Respiratory Infection due to SARS-CoV-2 in Individuals Aged 20 Years and Older–Fourth Update. Available online: https://www.ecdc.europa.eu/en/publications-data/interim-analysis-covid-19-vaccine-effectiveness-against-severe-acute-respiratory (accessed on 21 June 2023).
- Wu, F.; Liu, M.; Wang, A.; Lu, L.; Wang, Q.; Gu, C.; Chen, J.; Wu, Y.; Xia, S.; Ling, Y.; et al. Evaluating the Association of Clinical Characteristics with Neutralizing Antibody Levels in Patients Who Have Recovered from Mild COVID-19 in Shanghai, China. JAMA Intern. Med. 2020, 180, 1356–1362, Erratum in JAMA Intern. Med. 2020, 180, 1405. [Google Scholar] [CrossRef]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020, 92, 512–517. [Google Scholar] [CrossRef]
- Hojjat Jodaylami, M.; Djaïleb, A.; Ricard, P.; Lavallée, É.; Cellier-Goetghebeur, S.; Parker, M.F.; Coutu, J.; Stuible, M.; Gervais, C.; Durocher, Y.; et al. Cross-reactivity of antibodies from non-hospitalized COVID-19 positive individuals against the native, B.1.351, B.1.617.2, and P.1 SARS-CoV-2 spike proteins. Sci. Rep. 2021, 11, 21601, Erratum in Sci. Rep. 2021, 11, 22912. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-e18. [Google Scholar] [CrossRef]
- Tauzin, A.; Gong, S.Y.; Beaudoin-Bussières, G.; Vézina, D.; Gasser, R.; Nault, L.; Marchitto, L.; Benlarbi, M.; Chatterjee, D.; Nayrac, M.; et al. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe 2022, 30, 97–109.e5. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P. Extended interval BNT162b2 vaccination enhances peak antibody generation. npj Vaccines 2022, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Sansone, E.; Collatuzzo, G.; Renzetti, S.; Ditano, G.; Bonfanti, C.; Sala, E.; Terlenghi, L.; Matteelli, A.; Abedini, M.; Asafo, S.S.; et al. The Effect of the Immunization Schedule and Antibody Levels (Anti-S) on the Risk of SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers in Northern Italy. Vaccines 2023, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Visci, G.; Zunarelli, C.; Mansour, I.; Porru, S.; Palma, G.D.; Duval, X.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Lippi, G.; et al. Serological response after SARS-CoV2 vaccination in healthcare workers: A multicenter study. Med. Lav. 2022, 113, e2022022. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Sughayer, M.A.; Souan, L.; Abu Alhowr, M.M.; Al Rimawi, D.; Siag, M.; Albadr, S.; Owdeh, M.; Al Atrash, T. Comparison of the effectiveness and duration of anti-RBD SARS-CoV-2 IgG antibody response between different types of vaccines: Implications for vaccine strategies. Vaccine 2022, 40, 2841–2847. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, W.; Li, J.; Chen, M.; Li, Q.; Lv, M.; Zhou, S.; Bai, S.; Wang, Y.; Zhang, L.; et al. Status of Humoral and Cellular Immune Responses within 12 Months following CoronaVac Vaccination against COVID-19. mBio 2022, 13, e0018122. [Google Scholar] [CrossRef]
- Lustig, Y.; Gonen, T.; Meltzer, L.; Gilboa, M.; Indenbaum, V.; Cohen, C.; Amit, S.; Jaber, H.; Doolman, R.; Asraf, K.; et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat. Immunol. 2022, 23, 940–946. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Mizoue, T.; Tanaka, A.; Oshiro, Y.; Inamura, N.; Konishi, M.; Ozeki, M.; Miyo, K.; Sugiura, W.; Sugiyama, H.; et al. Sex-associated differences between BMI and SARS-CoV-2 antibody titers following the BNT162b2 vaccine. Obesity 2022, 30, 999–1003. [Google Scholar] [CrossRef] [PubMed]
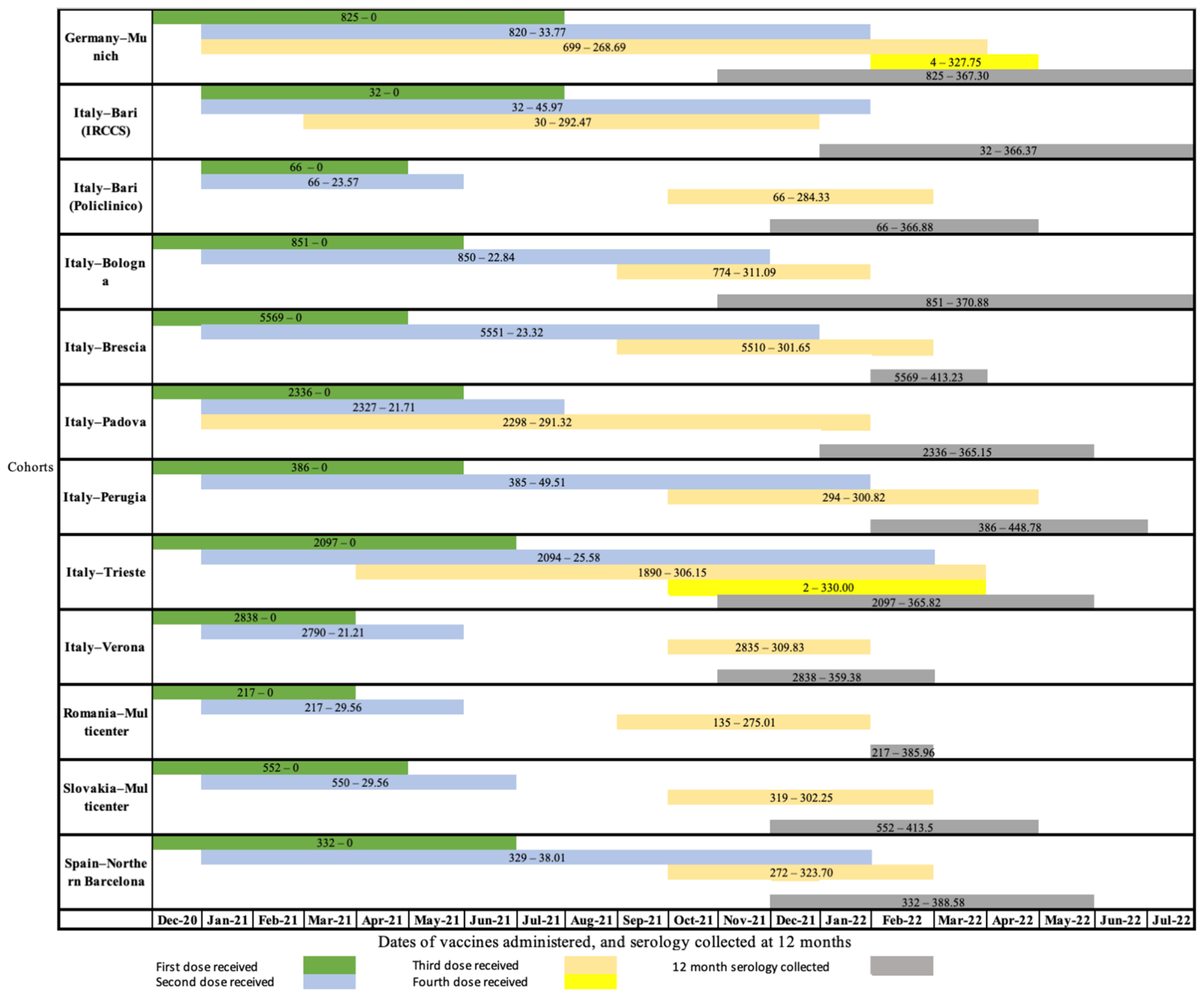
| Cohort | Assay | Crude Result: Mean (SE) * | Standardized Results: Mean (SE) * |
|---|---|---|---|
| Germany-Munich | Ro-RBD-Ig-quant-DBS# | 434.84 (13.85) | 4.39 (0.03) |
| Italy-Bari (IRCCS) | CLIA IgG quantitative | 6173.09 (1228.94) | 6.85 (0.18) |
| Italy-Bari (Policlinico) | Abbott SARS-CoV2 IgG II Quant Test | 41,379.33 (4459.53) | 8.05 (0.12) |
| Italy-Bologna | Ab anti SARS-CoV-2 S (RBD) IgG ECLIA | 2391.58 (14.69) | 2.36 (0.03) † |
| Italy-Brescia | ECLIA Elecsys® anti SARS CoV2 S for anti-SARS-CoV-2-S total antibody detection (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) | 4582.78 (12.58) | 1.42 (0.01) † |
| Italy-Padova | LIAISON SARS-CoV-2 TrimericS IgG | 7187.21 (139.77) | 10.40 (0.02) |
| Italy-Perugia | Diasorin | 1884.96 (21.34) | 2.00 (0.05) † |
| Italy-Trieste | CMIA Abbott anti S-RBD | 29,817.64 (1247.10) | 5.99 (0.02) |
| Italy-Verona | DIASORIN LIAISON® SARS-COV-2 TRIMERIC–S–IGG | 8515.69 (122.20) | 11.90 (0.02) |
| Romania-Multicenter | Abbot SARS-CoV-2 IgG II Quant test | 12,844.52 (910.43) | 6.53 (0.07) |
| Slovakia-Multicenter | Anti-SARS-CoV-2 QuantiVac ELISA (IgG) EUROIMMUN | 1330.16 (16.46) | 1.45 (0.04) † |
| Spain-Northern Barcelona region | DECOV1901 ELISA (IgG-S) | 3313.32 (76.24) | 15.54 (0.05) |
| Germany-Munich | Italy-Bari (IRCCS) | Italy-Bari (Policlinico) | Italy-Bologna | Italy-Brescia | Italy-Padova | Italy-Perugia | Italy-Trieste | Italy-Verona | Romania-Multicenter | Slovakia-Multicenter | Spain-Northern Barcelona | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 825 | 32 | 66 | 851 | 5569 | 2336 | 386 | 2097 | 2838 | 217 | 552 | 332 |
| Qualitative characteristics (N, (%)) | ||||||||||||
| Sex | ||||||||||||
| Male | 191 (23) | 10 (31) | 17 (26) | 239 (28) | 1426 (26) | 553 (24) | 94 (24) | 665 (32) | 648 (23) | 38 (18) | 88 (16) | 67 (20) |
| Female | 634 (77) | 22 (69) | 49 (74) | 612 (72) | 4143 (74) | 1783 (76) | 292 (76) | 1432 (68) | 2190 (77) | 179 (82) | 464 (84) | 265 (80) |
| Age group | ||||||||||||
| <=29 | 133 (16) | 17 (53) | 13 (20) | 84 (10) | 773 (14) | 188 (8) | 4 (1) | 196 (9) | 359 (13) | 10 (5) | 58 (11) | 24 (7) |
| 30–39 | 212 (26) | 2 (6) | 18 (27) | 201 (24) | 1005 (18) | 482 (21) | 42 (11) | 403 (19) | 526 (19) | 20 (9) | 72 (13) | 44 (13) |
| 40–49 | 170 (21) | 4 (13) | 14 (21) | 186 (22) | 1440 (26) | 484 (21) | 86 (22) | 467 (22) | 715 (25) | 62 (29) | 187 (34) | 120 ((36) |
| >=50 | 309 (38) | 9 (28) | 21 (32) | 380 (45) | 2351 (42) | 1182 (51) | 252 (66) | 1031 (49) | 1238 (44) | 125 (58) | 235 (43) | 144 (43) |
| Job title | ||||||||||||
| Physician | NA | 1 (3) | 20 (30) | 172 (20) | 1297 (23) | 515 (22) | 64 (17) | 468 (22) | 632 (22) | 96 (44) | 73 (13) | 118 (36) |
| Technician | NA | 4 (12) | 4 (6) | 102 (12) | 483 (9) | 155 (7) | 62 (16) | 311 (15) | 293 (10) | 59 (27) | 38 (7) | NA |
| Nurse | NA | 20 (62) | 24 (36) | 345 (41) | 2042 (37) | 1245 (53) | 46 (12) | 775 (37) | 1182 (42) | 29 (13) | 194 (35) | 139 (43) |
| Administration | NA | NA | 3 (5) | 40 (5) | 681 (12) | 80 (3) | 24 (6) | 124 (6) | 214 (8) | 24 (11) | 61 (11) | 45 (14) |
| Other HCW | NA | 7 (22) | 15 (23) | 191 (22) | 1066 (19) | 340 (15) | 185 (49) | 419 (20) | 517 (18) | 9 (4) | 185 (34) | 22 (7) |
| Previous SARS-CoV-2 infection (PCR and/or AntiN) | ||||||||||||
| Never infected | 550 (67) | 30 (94) | 3 (5) | 763 (90) | 3657 (66) | 2025 (87) | 340 (88) | 1543 (74) | 2347 (83) | 168 (77) | 258 (47) | 112 (34) |
| Infected at least once | 275 (33) | 2 (6) | 63 (95) | 88 (10) | 1912 (34) | 311 (13) | 46 (12) | 554 (26) | 491 (17) | 49 (23) | 294 (53) | 220 (66) |
| Type of vaccine | ||||||||||||
| Only Comirnaty [BioNTech/Pfizer] | 494 (60) | 32 (100) | 66 (100) | 821 (96) | 4675 (84) | 2211 (99.8) | 386 (100) | 1962 (96) | 2838 (100) | 208 (96) | 529 (96) | 107 (32) |
| Spikevax [Moderna] alone or with other ** vaccines | 315 (38) | 0 (0) | 0 (0) | 30 (4) | 891 (16) | 4 (0.2) | 0 (0) | 80 (4) | 0 (0) | 5 (2) | 12 (2) | 225 (68) |
| Comirnaty [BioNTech/Pfizer] with other vaccines *** (except Spikevax [Moderna]) | 16 (2) | 0 (0) | 0 (0) | 0 (0) | 3 (0.1) | 1 (0.1) | 0 (0) | 4 (0.2) | 0 (0) | 4 (2) | 11 (2) | NA |
| Number of vaccine doses | ||||||||||||
| 1 or 2 doses | 129 (16) | 2 (6) | 0 (0) | 77 (9) | 76 (1) | 47 (2) | 92 (24) | 207 (10) | 50 (2) | 82 (38) | 233 (42) | 60 (18) |
| 3 or 4 doses | 692 (84) | 30 (94) | 66 (100) | 774 (91) | 5493 (99) | 2289 (98) | 294 (76) | 1888 (90) | 2788 (98) | 135 (62) | 319 (58) | 272 (82) |
| Quantitative characteristics | ||||||||||||
| Standardized quantitative serology at 12 months | ||||||||||||
| Mean (SD) | 4.39 (0.03) | 6.85 (0.18) | 8.05 (0.12) | 2.36 (0.03) * | 1.42 (0.01) * | 10.40 (0.02) | 2.00 (0.05) * | 5.99 (0.02) | 11.90 (0.02) | 6.53 (0.07) | 1.45 (0.04) * | 15.54 (0.05) |
| Days between last dose and serology at 12 months | ||||||||||||
| Range | (1, 388) | (29, 286) | (28, 141) | (1, 517) | (6, 414) | (10, 420) | (29, 470) | (1, 435) | (10, 350) | (9, 392) | (20, 424) | (4, 396) |
| Mean (SD) | 130.95 (3.02) | 79.19 (10.82) | 82.54 (2.67) | 84.97 (3.01) | 113.78 (0.41) | 78.23 (0.91) | 192.99 (5.28) | 84.06 (1.88) | 49.86 (0.26) | 206.49 (8.34) | 226.13 (5.58) | 106.19 (5.41) |
| Days between last dose and serology at 12 months (30-day increase) § | ||||||||||||
| Range | (1, 13) | (1, 10) | (1, 5) | (1, 17) | (1, 14) | (1, 14) | (1, 16) | (1, 15) | (1, 12) | (1, 14) | (1, 15) | (1, 14) |
| Mean (SD) | 4.86 (0.10) | 3.12 (0.36) | 3.22 (0.10) | 3.36 (0.10) | 4.30 (0.01) | 3.08 (0.03) | 6.94 (0.18) | 3.29 (0.06) | 2.13 (0.01) | 7.44 (0.28) | 8.04 (0.18) | 4.03 (0.18) |
| Characteristics [Cohorts Included *] | RR | 95% CI |
|---|---|---|
| Gender 1 [all] | ||
| Male | 1.00 | Ref |
| Female | 0.89 | 0.79–1.01 |
| Age 1 [all] | ||
| 10-year increase | 1.04 | 1.00–1.08 |
| Days between last vaccine dose and 12-month serology 1 [all] | ||
| 30-day increase | 0.94 | 0.91–0.98 |
| Previous SARS-CoV-2 infection (detection: PCR/antiN serology test) 1 [all] | ||
| Never infected | 1.00 | Ref |
| Infected at least once | 1.78 | 1.29–2.45 |
| Number of doses ¹ [Ge-Mu, It-Bo, It-Br, It-Pa, It-Pe, It-Ts, It-Vr, Ro-Mc, Sk-Mc, Sp-Ba] | ||
| 1–2 | 1.00 | Ref |
| 3–4 | 1.41 | 0.86–2.32 |
| Job title ¹ [It-Ba(I), It-Ba(II), It-Bo, It-Br, It-Pa, It-Pe, It-Ts, It-Vr, Ro-Mc, Sk-Mc, Sp-Ba] | ||
| Physician, including resident | 1.00 | Ref |
| Nurse | 1.05 | 0.96–1.13 |
| Technician | 1.08 | 0.97–1.19 |
| Administration | 1.09 | 1.02–1.16 |
| Other, including auxiliary workers | 1.01 | 0.94–1.08 |
| Type of vaccine [Ge-Mu, It-Bo, It-Br, It-Pa, It-Ts, Ro-Mc, Sk-Mc, Sp-Ba] | ||
| Only Comirnaty [Pfizer/BioNTech] | 1.00 | Ref |
| Spikevax [Moderna] alone or with other vaccines | 1.07 | 0.97–1.19 |
| Comirnaty [Pfizer/BioNTech] with other vaccines (except Spikevax [Moderna]) | 0.77 | 0.60–0.98 |
| Previous SARS-CoV-2 infection (detection: PCR) [all] | ||
| Never infected | 1.00 | Ref |
| Infected before vaccination | 1.35 | 0.98–1.85 |
| Infected after 1st dose of vaccine | 2.80 | 1.64–4.77 |
| Infected at both times | 1.82 | 0.87–3.77 |
| Previous SARS-CoV-2 infection (detection: antiN serology test) ** [ Ge-Mu, It-Br, Sp-Ba] | ||
| Never infected | 1.00 | Ref |
| Infected at least once | 1.46 | 1.00–2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leomanni, L.; Collatuzzo, G.; Sansone, E.; Sala, E.; De Palma, G.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Basso, D.; Pavanello, S.; et al. Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Vaccines 2023, 11, 1527. https://doi.org/10.3390/vaccines11101527
Leomanni L, Collatuzzo G, Sansone E, Sala E, De Palma G, Porru S, Spiteri G, Monaco MGL, Basso D, Pavanello S, et al. Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Vaccines. 2023; 11(10):1527. https://doi.org/10.3390/vaccines11101527
Chicago/Turabian StyleLeomanni, Ludovica, Giulia Collatuzzo, Emanuele Sansone, Emma Sala, Giuseppe De Palma, Stefano Porru, Gianluca Spiteri, Maria Grazia Lourdes Monaco, Daniela Basso, Sofia Pavanello, and et al. 2023. "Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project" Vaccines 11, no. 10: 1527. https://doi.org/10.3390/vaccines11101527
APA StyleLeomanni, L., Collatuzzo, G., Sansone, E., Sala, E., De Palma, G., Porru, S., Spiteri, G., Monaco, M. G. L., Basso, D., Pavanello, S., Scapellato, M. L., Larese Filon, F., Cegolon, L., Mauro, M., Lodi, V., Lazzarotto, T., Noreña, I., Reinkemeyer, C., Giang, L. T. T., ... Boffetta, P. (2023). Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Vaccines, 11(10), 1527. https://doi.org/10.3390/vaccines11101527














