Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Analysis
3. Results
3.1. Identification of Studies
3.2. Characteristics of Included Studies
3.3. Study Quality
3.4. Meta-Analysis
3.4.1. Effects of BCG on Non-Tuberculosis Respiratory Infections and COVID-19
3.4.2. Effects of BCG on Infections of Any Origin and Sepsis
3.4.3. Effects of BCG on Mortality
3.4.4. Effects of BCG on Hospitalization
3.4.5. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BCG vaccine: WHO position paper, February 2018 Recommendations. Vaccine 2018, 36, 3408–3410. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Soares-Weiser, K.; López-López, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Aaby, P.; Napirna, B.M.; Roth, A.; Ravn, H.; Rodrigues, A.; Whittle, H.; Benn, C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guéerin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012, 31, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Aaby, P.; Lund, N.; Monteiro, I.; Jensen, K.J.; Eriksen, H.B.; Schaltz-Buchholzer, F.; Jørgensen, A.S.P.; Rodrigues, A.; Fisker, A.B.; et al. Early BCG-Denmark and Neonatal Mortality among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 65, 1183–1190. [Google Scholar] [CrossRef]
- Kjærgaard, J.; Birk, N.M.; Nissen, T.N.; Thøstesen, L.M.; Pihl, G.T.; Benn, C.S.; Jeppesen, D.L.; Pryds, O.; Kofoed, P.E.; Aaby, P.; et al. Nonspecific effect of BCG vaccination at birth on early childhood infections: A randomized, clinical multicenter trial. Pediatr. Res. 2016, 80, 681–685. [Google Scholar] [CrossRef]
- Messina, N.L.; Pittet, L.F.; Gardiner, K.; Freyne, B.; Francis, K.L.; Zufferey, C.; Abruzzo, V.; Morrison, C.; Allen, K.J.; Flanagan, K.L.; et al. Neonatal Bacille Calmette-Guérin Vaccination and Infections in the First Year of Life: The MIS BAIR Randomized Controlled Trial. J. Infect. Dis. 2021, 224, 1115–1127. [Google Scholar] [CrossRef]
- Aaby, P.; Benn, C.S.; Flanagan, K.L.; Klein, S.L.; Kollmann, T.R.; Lynn, D.J.; Shann, F. The non-specific and sex-differential effects of vaccines. Nat. Rev. Immunol. 2020, 20, 464–470. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.M.; et al. Long-Lasting Effects of BCG Vaccination on Both Heterologous Th1/Th17 Responses and Innate Trained Immunity. J. Innate. Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.; Harbeson, D.J.; Shannon, C.P.; Cai, B.; He, D.; Ben-Othman, R.; Francis, F.; Huang, J.; Varankovich, N.; Liu, A.; et al. BCG vaccination–induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl. Med. 2020, 12, eaax4517. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A Small Jab A Big Effect: Nonspecific Immunomodulation By Vaccines. Trends. Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Boutron, I.; Chaimani, A.; Meerpohl, J.J.; Hróbjartsson, A.; Devane, D.; Rada, G.; Tovey, D.; Grasselli, G.; Ravaud, P.; COVID-NMA Consortium. The COVID-NMA Project: Building an Evidence Ecosystem for the COVID-19 Pandemic. Ann. Intern. Med. 2020, 173, 1015–1017. [Google Scholar] [CrossRef]
- Tsilika, M.; Taks, E.; Dolianitis, K.; Kotsaki, A.; Leventogiannis, K.; Damoulari, C.; Kostoula, M.; Paneta, M.; Adamis, G.; Papanikolaou, I.; et al. Activate-2: A Double-Blind Randomized Trial of BCG Vaccination against COVID-19 in Individuals at Risk. Front. Immunol. 2022, 13, 873067. [Google Scholar] [CrossRef]
- Borissov, N.; Haas, Q.; Minder, B.; Kopp-Heim, D.; von Gernler, M.; Janka, H.; Teodoro, D.; Amini, P. Reducing systematic review burden using Deduklick: A novel, automated, reliable, and explainable deduplication algorithm to foster medical research. Syst. Rev. 2022, 11, 172. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions. 2021. Available online: https://training.cochrane.org/handbook/current (accessed on 25 November 2021).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.L. Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. J. Intern. Med. 2020, 288, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.J.C.F.M.; Taks, E.; Doesschate, T.; van der Vaart, T.W.; Janssen, A.B.; Müller, L.; Ostermann, P.; Dijkstra, H.; Lemmers, H.; Simonetti, E.; et al. Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults During the Coronavirus Disease 2019 Pandemic. Clin. Infect. Dis. 2022, 75, e938–e946. [Google Scholar] [CrossRef] [PubMed]
- Czajka, H.; Zapolnik, P.; Krzych, Ł.; Kmiecik, W.; Stopyra, L.; Nowakowska, A.; Jackowska, T.; Darmochwał-Kolarz, D.; Szymański, H.; Radziewicz-Winnicki, I.; et al. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-Vaccination on the Incidence and Severity of SARS-CoV-2 Infections among Symptomatic Healthcare Professionals during the COVID-19 Pandemic in Poland—First Results. Vaccines 2022, 10, 314. [Google Scholar] [CrossRef]
- Villanueva, P.; Wadia, U.; Crawford, N.W.; Messina, N.L.; Kollmann, T.R.; Lucas, M.; Manning, L.; Richmond, P.; Pittet, L.F.; Curtis, N. The safety of co-administration of Bacille Calmette-Guérin (BCG) and influenza vaccines. Fast PE, editor. PLoS ONE 2022, 17, e0268042. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.; Falfán-Valencia, R.; Pérez-Rubio, G.; Andrade, W.A.; Rojas-Serrano, J.; Ambrocio-Ortiz, E.; Galicia-Álvarez, D.S.; Bárcenas-Montiel, I.; Velasco-Medina, A.; Velázquez-Sámano, G. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells 2021, 10, 3179. [Google Scholar] [CrossRef]
- Schaltz-Buchholzer, F.; Aaby, P.; Monteiro, I.; Camala, L.; Faurholt Simonsen, S.; Nørtoft Frankel, H.; Lindberg Larsen, K.; Golding, C.N.; Kollmann, T.R.; Amenyogbe, N.; et al. Immediate Bacille Calmette-Guérin Vaccination to Neonates Requiring Perinatal Treatment at the Maternity Ward in Guinea-Bissau: A Randomized Controlled Trial. J. Infect. Dis. 2021, 224, 1935–1944. [Google Scholar] [CrossRef]
- Sinha, S.; Ajayababu, A.; Thukral, H.; Gupta, S.; Guha, S.K.; Basu, A.; Gupta, G.; Thakur, P.; Lingaiah, R.; Das, B.K.; et al. Efficacy of Bacillus Calmette–Guérin (BCG) Vaccination in Reducing the Incidence and Severity of COVID-19 in High-Risk Population (BRIC): A Phase III, Multi-centre, Quadruple-Blind Randomised Control Trial. Infect. Dis. Ther. 2022, 11, 2205–2217. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, n71. [Google Scholar] [CrossRef]
- Jayaraman, K.; Adhisivam, B.; Nallasivan, S.; Krishnan, R.G.; Kamalarathnam, C.; Bharathi, M.; McSharry, B.; Namachivayam, S.P.; Shann, F.; Boopalan, S.I.; et al. Two randomized trials of the effect of the russian strain of bacillus calmette-guérin alone or with oral polio vaccine on neonatal mortality in infants weighing < 2000 g in india. Pediatr. Infect. Dis. J. 2019, 38, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.W.; Netea, M.G.; Dockrell, H.M.; et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: An investigator-blind randomised controlled trial. Lancet. Infect. Dis. 2021, 21, 993–1003. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L634355118&from=export (accessed on 25 November 2021). [CrossRef] [PubMed]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. Tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- dos Anjos, L.R.B.; da Costa, A.C.; da Rocha Oliveira Cardoso, A.; Guimarães, R.A.; Rodrigues, R.L.; Ribeiro, K.M.; Borges, K.C.M.; de Oliveira Carvalho, A.C.; Dias, C.I.S.; de Oliveira Rezende , A.; et al. Efficacy and Safety of BCG Revaccination with M. bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front. Immunol. 2022, 13, 841868. [Google Scholar] [CrossRef] [PubMed]
- Doesschate, T.; van der Vaart, T.W.; Debisarun, P.A.; Taks, E.; Moorlag, S.J.C.F.M.; Paternotte, N.; Boersma, W.G.; Kuiper, V.P.; Roukens, A.H.E.; Rijnders, B.J.A.; et al. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 2022, 28, 1278–1285. [Google Scholar] [CrossRef]
- Upton, C.M.; Wijk, R.C.; van Mockeliunas, L.; Simonsson, U.S.H.; McHarry, K.; van den Hoogen, G.; Muller, C.; von Delft, A.; van der Westhuizen, H.-M.; van Crevel, R.; et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. eClinicalMedicine 2022, 48, 101414. [Google Scholar] [CrossRef]
- Wardhana Datau, E.A.; Sultana, A.; Mandang, V.V.; Jim, E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta. Medica. Indones. 2011, 43, 185–190. [Google Scholar]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.E.; Damoraki, G.; et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 2020, 183, 315–323.e9. [Google Scholar] [CrossRef]
- Blossey, A.M.; Brückner, S.; May, M.; Parzmair, G.P.; Sharma, H.; Shaligram, U.; Grode, L.; Kaufmann, S.H.E.; Netea, M.G.; Schindler, C. VPM1002 as Prophylaxis Against Severe Respiratory Tract Infections Including Coronavirus Disease 2019 in the Elderly: A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Study. Clin. Infect. Dis. 2022, ciac881. [Google Scholar] [CrossRef]
- Glynn, J.R.; Dube, A.; Fielding, K.; Crampin, A.C.; Karonga Prevention Trial Group; Kanjala, C.; Fine, P.E.M. The effect of BCG revaccination on all-cause mortality beyond infancy: 30-year follow-up of a population-based, double-blind, randomised placebo-controlled trial in Malawi. Lancet Infect. Dis. 2021, 21, 1590–1597. [Google Scholar] [CrossRef]
- Kjærgaard, J. Bacillus Calmette-Guérin vaccination at birth: Effects on early childhood infections, growth, and development. Dan. Med. J. 2016, 63, B5304. [Google Scholar] [PubMed]
- Schaltz-Buchholzer, F.; Biering-Sorensen, S.; Lund, N.; Monteiro, I.; Umbasse, P.; Fisker, A.B.; Andersen, A.; Rodrigues, A.; Aaby, P.; Benn, C.S. Early Bacille Calmette-Guerin vaccination, hospitalizations and hospital deaths: Analysis of a secondary outcome in three randomized trials from Guinea-Bissau. J. Infect. Dis. 2018, 219, 624–632. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01954078/full (accessed on 25 November 2021). [CrossRef]
- Kjærgaard, J.; Stensballe, L.G.; Birk, N.M.; Nissen, T.N.; Thøstesen, L.M.; Pihl, G.T.; Nielsen, A.V.; Kofoed, P.E.; Aaby, P.; Pryds, O.; et al. Bacillus Calmette-Guérin vaccination at birth: Effects on infant growth. A randomized clinical trial. Early. Hum. Dev. 2016, 100, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Prentice, S.; Dockrell, H.M. BCG Specific and Nonspecific Effects: Different Questions, Similar Challenges. J. Infect. Dis. 2021, 224, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, I.; Aaby, P.; Jensen, H. Routine vaccinations and child survival: Follow up study in Guinea-Bissau, West Africa. BMJ 2000, 321, 1435–1438. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 –- conclusions and recommendations. Releve. Epidemiol. Hebd. 2014, 89, 221–236. [Google Scholar]
- Faustman, D.L.; Lee, A.; Hostetter, E.R.; Aristarkhova, A.; Ng, N.C.; Shpilsky, G.F.; Tran, L.; Wolfe, G.; Takahashi, H.; Dias, H.F.; et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep. Med. 2022, 3, 100728. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef]
- Bannister, S.; Sudbury, E.; Villanueva, P.; Perrett, K.; Curtis, N. The safety of BCG revaccination: A systematic review. Vaccine 2021, 39, 2736–2745. [Google Scholar] [CrossRef]
- EClinicalMedicine. Antimicrobial resistance: A top ten global public health threat. eClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef]
- Manesh, A.; Varghese, G.M. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe. 2021, 2, e419–e420. [Google Scholar] [CrossRef] [PubMed]


| Lead Author, Publication Date | Location | Study Period | Age Group | Intervention (BCG Type) | Time of Intervention | Control | Follow-Up | Total Trial Participants | Health Status |
|---|---|---|---|---|---|---|---|---|---|
| Aaby et al., 2011 Schaltz-Buchholzer et al., 2013 Schaltz-Buchholzer et al., 2018 | Guinea-Bissau | Nov 2002–Mar 2008 | newborn children | BCG Denmark | immediately after birth | BCG later, i.e., when the child had gained weight or at 6 weeks of age | 12 months | 2320 | low birth weight children < 2500 g |
| Wardhana et al., 2011 | Indonesia | Jun 2009–Nov 2009 | age 60–75 years | BCG weakened living Mycobacterium bovis Pasteur Paris strain mp 1173-P2 produced by PT Biofarma, Bandung, Indonesia | once a month for 3 months in succession | Placebo | 6 months | 34 | healthy individuals |
| Biering-Sorensen et al., 2012 Schaltz-Buchholzer et al., 2018 | Guinea-Bissau | Nov 2004–Mar 2008 | newborn children | BCG Denmark | immediately after birth | BCG later, i.e., when the child had gained weight or at 6 weeks of age | 12 months | 104 | low birth weight children < 2500 g |
| Kjærgaard et al., 2016 Stensballe et al., 2017 Stensballe et al., 2019 | Denmark | Sep 2012–Jan 2015 | newborn children | BCG Denmark | within 7 days of age | no intervention | 13 months | 4262 | healthy newborns |
| Biering-Sorensen et al., 2017 Schaltz-Buchholzer et al., 2018 | Guinea-Bissau | Feb 2008–Sep 2013 | newborn children | BCG Denmark | immediately after birth | BCG later, i.e., when the child had gained weight or at 6 weeks of age | 12 months | 4154 | low birth weight children < 2500 g |
| Nemes et al., 2018 | South Africa | May 2015–Dec 2016 | age 12–17 years | BCG Denmark | revaccination on day 0 (participants were BCG vaccinated in infancy) | Placebo | 24 months | 989 | healthy individuals |
| Jayaraman et al., 2019 | India | Oct 2013–not reported | newborn children | BCG-Russia | immediately after birth | BCG later, i.e., 28 days after birth | 28 days | 3072 | low birth weight children < 2000 g |
| Giamarellos-Bourboulis et al., 2020 | Greece | Sep 2017–Nov 2020 | age ≥ 65 years | BCG Denmark | Day of hospital discharge | Placebo | 12 months | 198 | Elderly participants discharged from hospital with various comorbidities |
| Glynn et al., 2021 | Karonga District, northern Malawi | Enrollment of partcipants: Jan 1986–Nov 1989 | 3 months–75 years | BCG (Glaxo-strain) revaccination | revaccination after randomisation | Placebo | Northern area: 1991–1994 by active follow-up, Southern area: 2002–2018 by demographic surveillance | Northern area: 7389, Southern area: 5616 | Excluded were individuals with past or current leprosy or tuberculosis, severe malnutrition, or other severe illness. |
| Messina et al., 2021 | Australia | Aug 2013–Sep 2016 | newborn children | BCG Denmark | first 10 days of life | no intervention | 12 months | 1272 | healthy newborns |
| Prentice et al., 2021 | Uganda | Mar 2014–Jul 2015 | newborn children | BCG Denmark | immediately after birth | BCG later, i.e., 6 weeks after birth | 6 weeks | 560 | healthy infants |
| Tsilika et al. 2022 | Greece | May 2020–May 2021 | age ≥ 50 years; mean age 69 years | BCG Moscow | day of hospital discharge | Placebo | 6 months | 301 | Elderly participants discharged from hospital with various comorbidities |
| dos Anjos et al., 2022 | Brazil | Aug 2020–Aug 2021 | adult healthcare workers | BCG Moscow | revaccination after randomisation | no intervention | 180 days | 113 | healthy individuals |
| Doesschate et al., 2022 | Netherlands | Mar 2020–Apr 2021 | adult healthcare workers | BCG Denmark | (re-)vaccination after randomisation | Placebo | 1 year | 1511 | healthy individuals |
| Upton et al., 2022 | South Africa | Enrollment of participants: May 2020–Oct 2020 | adult healthcare workers | BCG Denmark | revaccination after randomisation | Placebo | 52 weeks | 265 (per-protocol analysis) | healthy individuals (48.5% with latent tuberculosis) |
| Blossey et al., 2022 | Germany | Jun 2020–Oct 2021 | age ≥ 60 years | VPM1002 | (re-)vaccination after randomisation | Placebo | 240 days | 2037 | healthy individuals |
| Author Name, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias * |
|---|---|---|---|---|---|---|---|
| Aaby et al., 2011 Schaltz-Buchholzer et al., 2013 Schaltz-Buchholzer et al., 2018 | |||||||
| Wardhana et al., 2011 | ///////////////// | ///////////////// | ///////////////// | ///////////////// | |||
| Biering-Sorensen et al., 2012 Schaltz-Buchholzer et al., 2018 | |||||||
| Kjærgaard et al., 2016 Stensballe et al., 2017 Stensballe et al., 2019 | ////////////// | ||||||
| Biering-Sorensen et al., 2017 Schaltz-Buchholzer et al., 2018 | ///////////////// | ////////////// | |||||
| Nemes et al., 2018 | |||||||
| Jayaraman et al., 2019 | |||||||
| Giamarellos-Bourboulis et al., 2020 | ////////////// | ||||||
| Glynn et al., 2021 | ///////////////// | ////////////// | |||||
| Messina et al., 2021 | ///////////////// | ////////////// | |||||
| Prentice et al., 2021 | |||||||
| Tsilika et al. 2022 | ///////////////// | ///////////////// | ////////////// | ||||
| Dos Anjos et al. 2022 | ////////////// | ||||||
| Doesschaete et al. 2022 | ///////////////// | ////////////// | |||||
| Upton et al. 2022 | ////////////// | ||||||
| Blossey et al., 2022 | ////////////// |
| Outcome | No. of Trials | No. of Study Participants | No. of Cases | Combined HR (95% CI) | Test for Heterogeneity |
|---|---|---|---|---|---|
| Respiratory infections | 9 | 8062 | 902 | 0.56 (0.39–0.82) | I2 = 77%; p < 0.0001 |
| COVID-19 | 5 | 2749 | 263 | 0.88 (0.68–1.14) | I2 = 41%; p = 0.15 |
| Infections of any origin | 4 | 6244 | 1298 | 0.84 (0.71–1.00) | I2 = 47%; p = 0.13 |
| Sepsis | 3 | 7293 | 117 | 0.78 (0.55–1.10) | I2 = 0%; p = 0.97 |
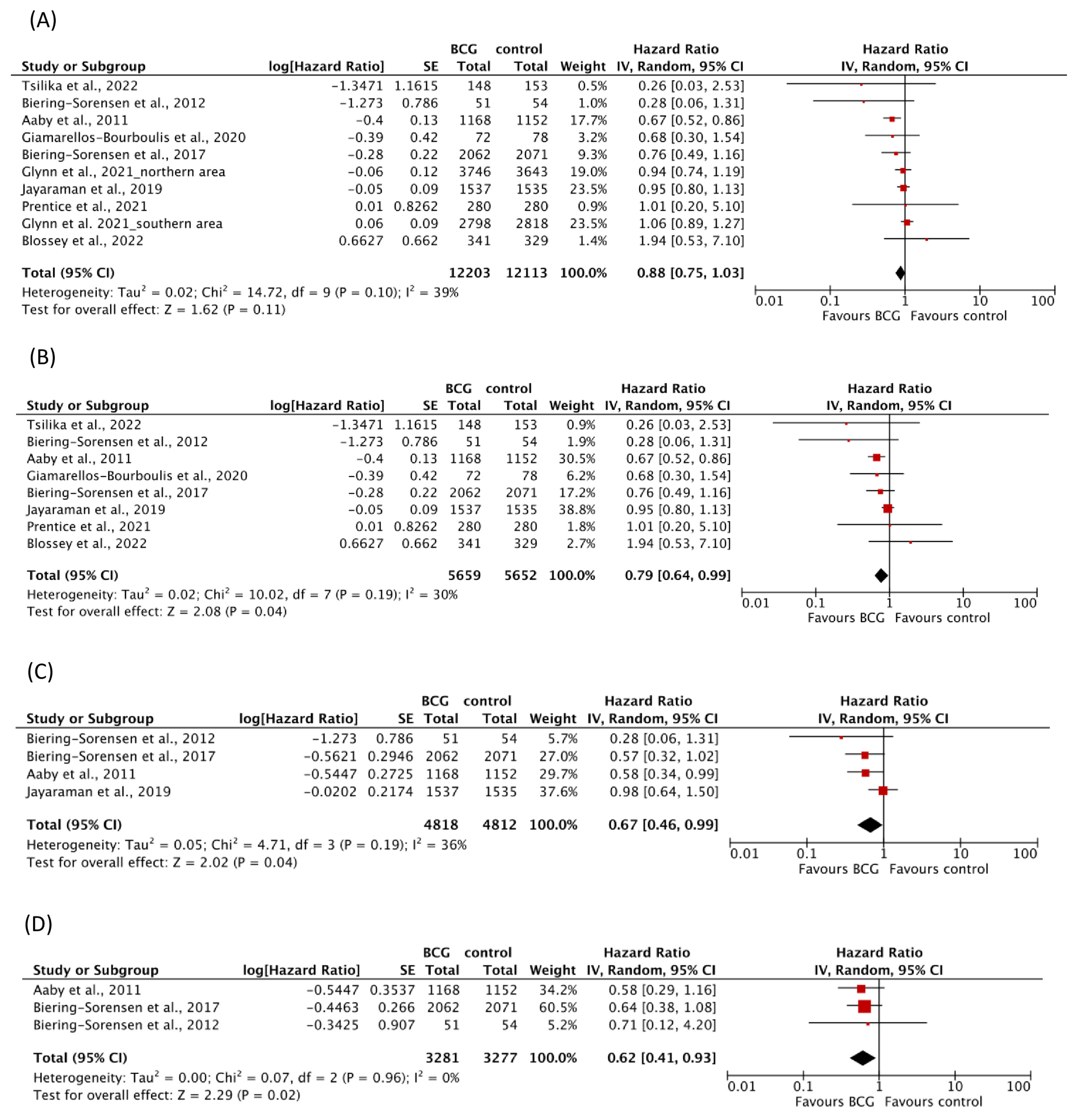
| Mortality | 9 | 24,316 | 1452 | 0.88 (0.75–1.03) | I2 = 39%; p = 0.10 |
| Mortality, follow-up ≤ 1 year | 8 | 11,311 | 936 | 0.79 (0.64–0.99) | I2 = 30%; p = 0.19 |
| Mortality for infections | 4 | 9630 | 194 | 0.67 (0.46–0.99) | I2 = 36%; p = 0.19 |
| Mortality for respiratory infections | 3 | 7123 | 16 | 0.47 (0.18–1.24) | I2 = 0%; p = 0.84 |
| Mortality for sepsis | 3 | 6558 | 94 | 0.62 (0.41–0.93) | I2 = 0%; p = 0.96 |
| Hospitalization | 9 | 13,367 | 2516 | 1.01 (0.91–1.11) | I2 = 0%; p = 0.70 |
| Hospitalization for infections | 3 | 12,117 | 886 | 0.96 (0.85–1.10) | I2 = 0%; p = 0.72 |
| Hospitalization for respiratory infections | 4 | 7708 | 45 | 0.64 (0.27–1.53) | I2 = 52%; p = 0.10 |
| Respiratory Infections | All-Cause Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup Heterogeneity | Test for Subgroup Differences | Subgroup Heterogeneity | Test for Subgroup Differences | |||||||||
| Variable | No. of Trials | HR [95% CI] | I2 [%] | p-Value | I2 [%] | p-Value | No. of Trials | HR [95% CI] | I2 [%] | p-Value | I2 [%] | p-Value |
| Age | ||||||||||||
| infants | 3 | 0.75 [0.58, 0.97] | 0 | 0.67 | 77 | <0.0001 | 5 | 0.79 [0.62, 1.00] | 44 | 0.13 | 39 | 0.1 |
| adolescents or adults | 6 | 0.43 [0.22, 0.83] | 85 | <0.00001 | 4 | 1.01 [0.88, 1.16] | 0 | 0.42 | ||||
| Health status | ||||||||||||
| low birth-weight children or morbid participants | 2 | 0.22 [0.10, 0.46] | 0 | 0.6 | 77 | <0.0001 | 6 | 0.81 [0.71, 0.92] | 52 | 0.06 | 49 | 0.04 |
| other | 7 | 0.69 [0.49, 0.97] | 73 | 0.001 | 3 | 1.02 [0.89, 1.18] | 0 | 0.66 | ||||
| Trial region | ||||||||||||
| Western Europe, Australia | 5 | 0.52 [0.30, 0.89] | 63 | 0.03 | 77 | <0.0001 | 3 | 0.84 [0.34, 2.04] | 31 | 0.24 | 39 | 0.1 |
| Africa, Indonesia, India, South America | 4 | 0.59 [0.33, 1.05] | 85 | 0.0001 | 6 | 0.88 [0.75, 1.04] | 49 | 0.07 | ||||
| Follow-up period | ||||||||||||
| ≤6 months | 3 | 0.35 [0.12, 1.07] | 69 | 0.04 | 77 | <0.0001 | 6 | 0.78 [0.61, 0.99] | 39 | 0.15 | 39 | 0.1 |
| >6 months | 6 | 0.61 [0.39, 0.97] | 80 | 0.0002 | 3 | 1.01 [0.88, 1.16] | 0 | 0.47 | ||||
| Method of outcome collection | ||||||||||||
| participant reported outcome without medical diagnosis | 5 | 0.79 [0.54, 1.15] | 62 | 0.03 | 77 | <0.0001 | 0 | - | - | - | - | - |
| medical diagnosed outcome | 4 | 0.37 [0.18, 0.75] | 72 | 0.01 | 9 | 0.88 [0.75, 1.03] | 39 | 0.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunk, G.; Davidović, M.; Bohlius, J. Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2023, 11, 121. https://doi.org/10.3390/vaccines11010121
Trunk G, Davidović M, Bohlius J. Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines. 2023; 11(1):121. https://doi.org/10.3390/vaccines11010121
Chicago/Turabian StyleTrunk, Gerhard, Maša Davidović, and Julia Bohlius. 2023. "Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Vaccines 11, no. 1: 121. https://doi.org/10.3390/vaccines11010121
APA StyleTrunk, G., Davidović, M., & Bohlius, J. (2023). Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines, 11(1), 121. https://doi.org/10.3390/vaccines11010121







