Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice
Abstract
1. Introduction
2. Materials and Methods
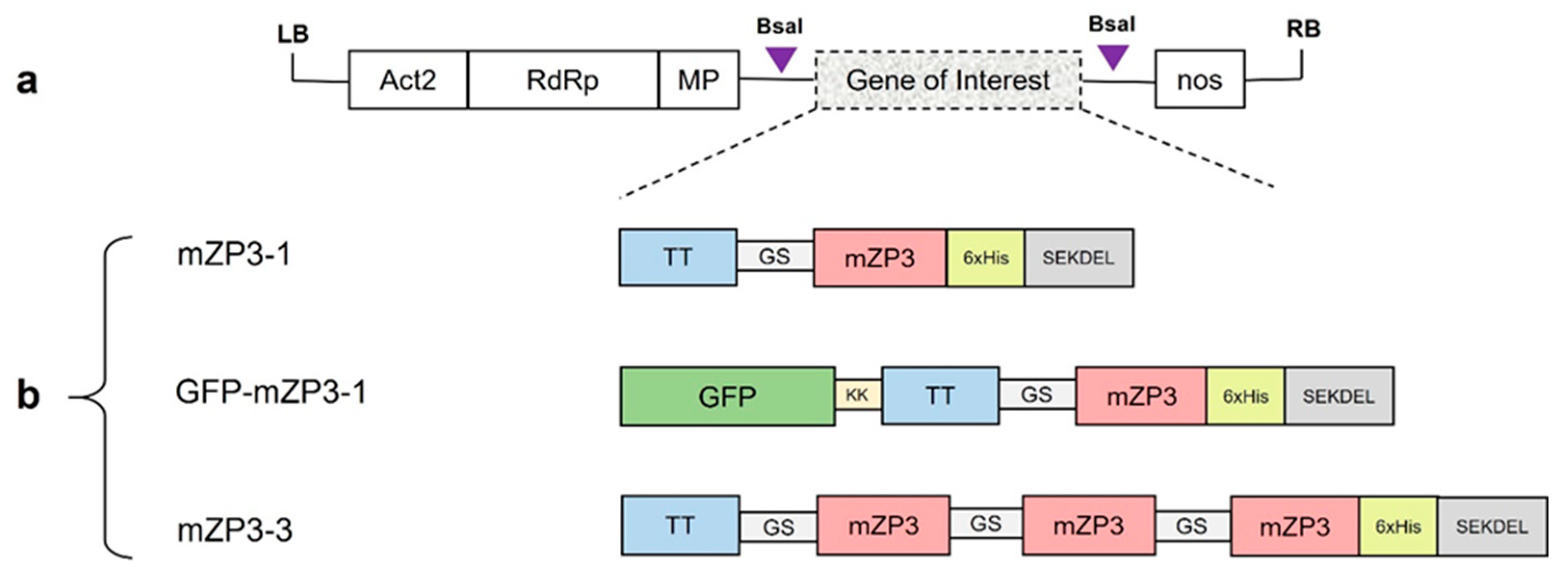
2.1. Construction of Plant Expression Vectors
2.2. Agroinfiltration Procedure
2.3. Total Soluble Protein Extraction from N. benthamiana Leaves
2.4. Ni-NTA Purification
2.5. SDS–PAGE and Western Blot Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
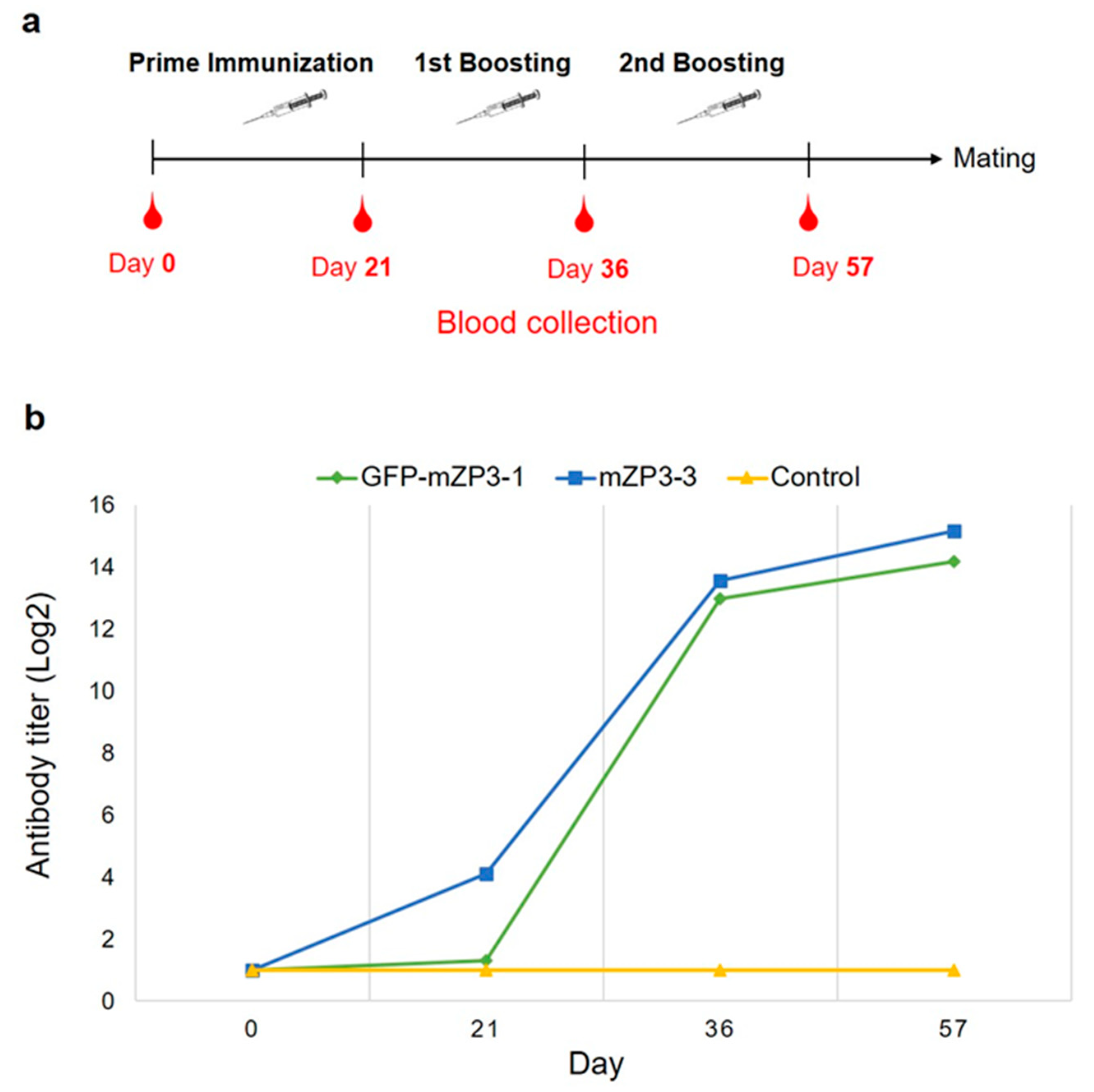
2.7. Subcutaneous Immunization of Mice
2.8. Detection of the Antibody Titer Using ELISA
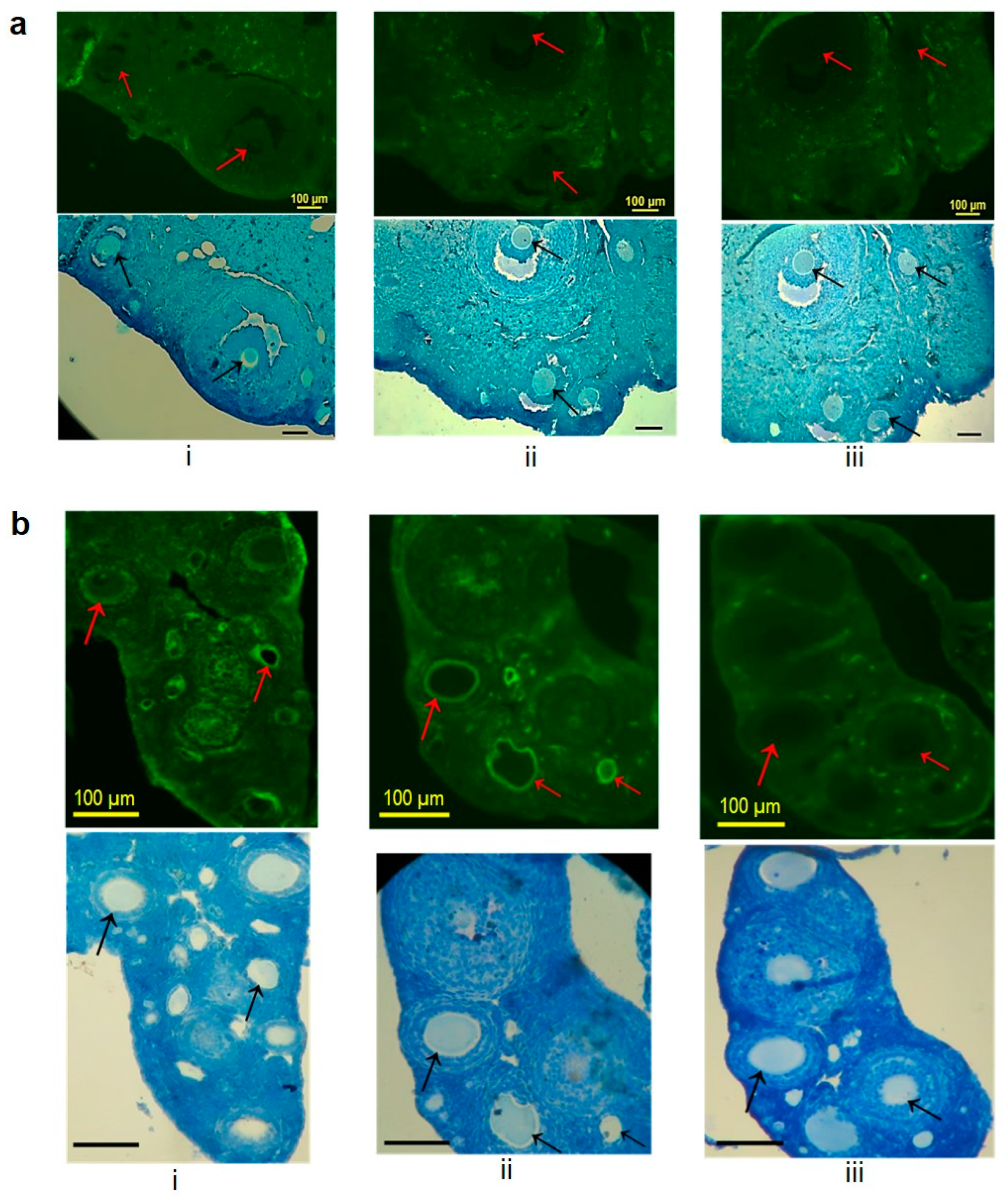
2.9. Indirect Immunofluorescence
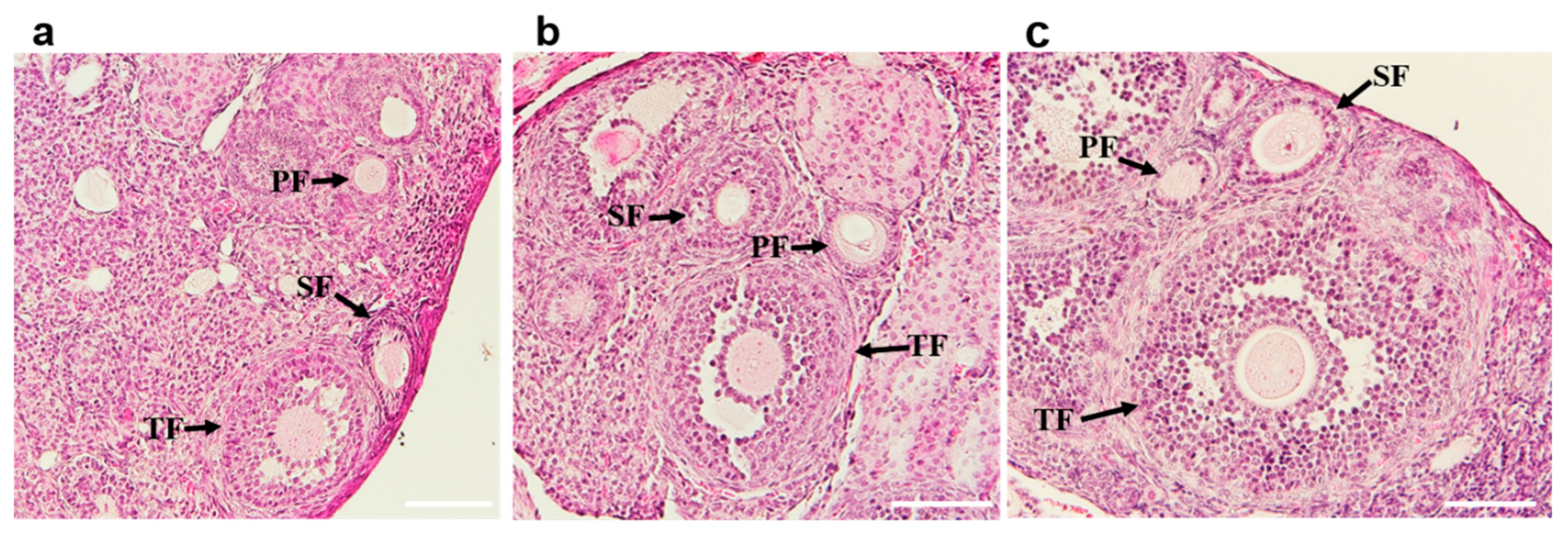
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
3.1. Construction of Expression Vectors for Transient Expression
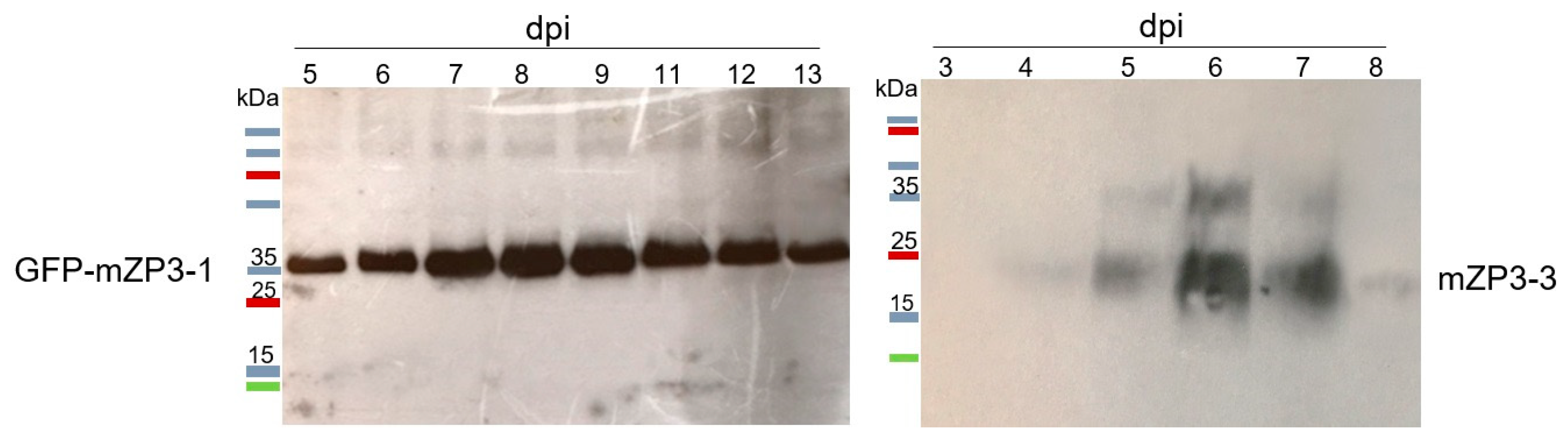
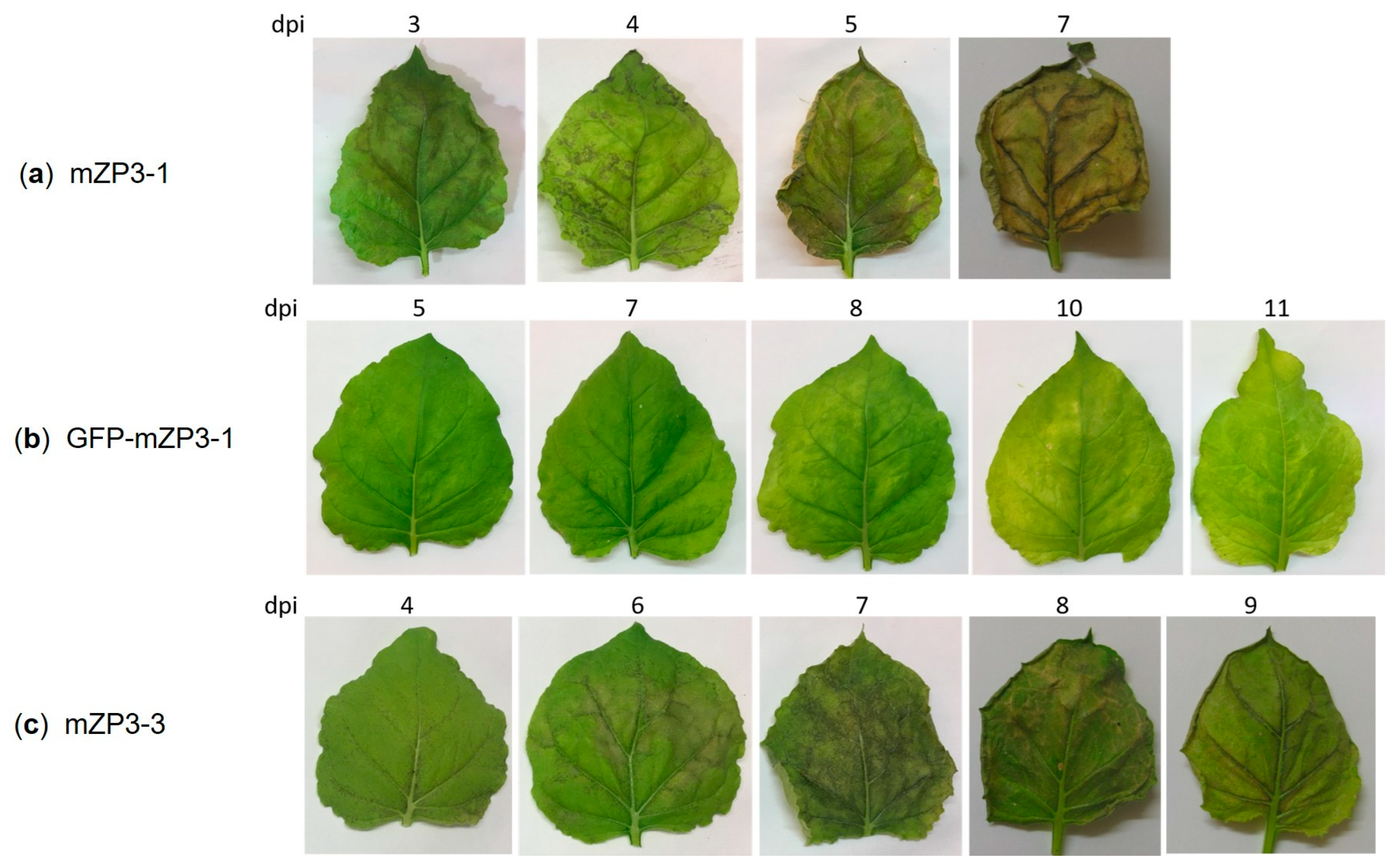
3.2. Optimization of mZP3 Expression in N. benthamiana
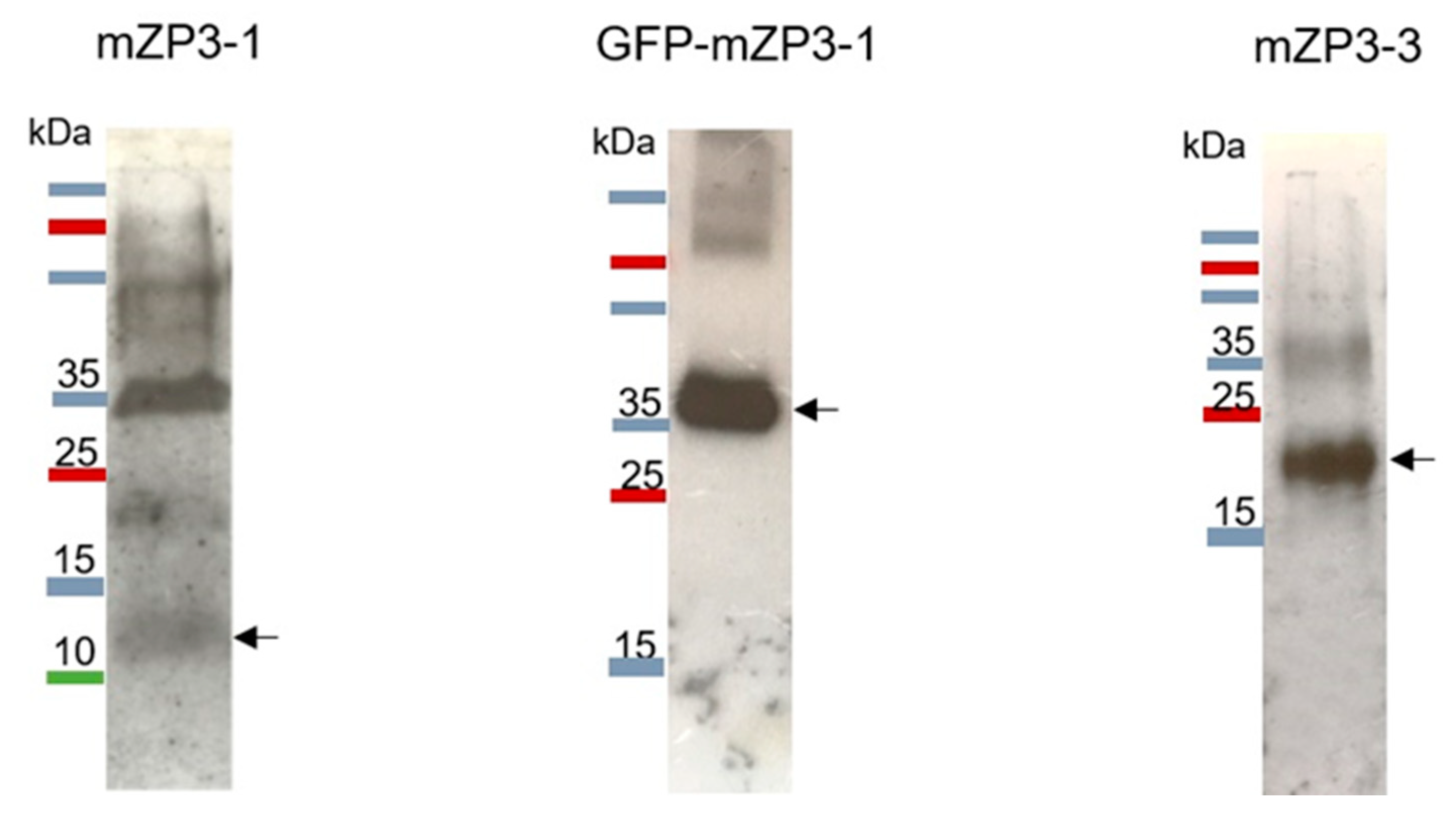
3.3. Purification and Characterization of Plant-Produced mZP3 Antigens
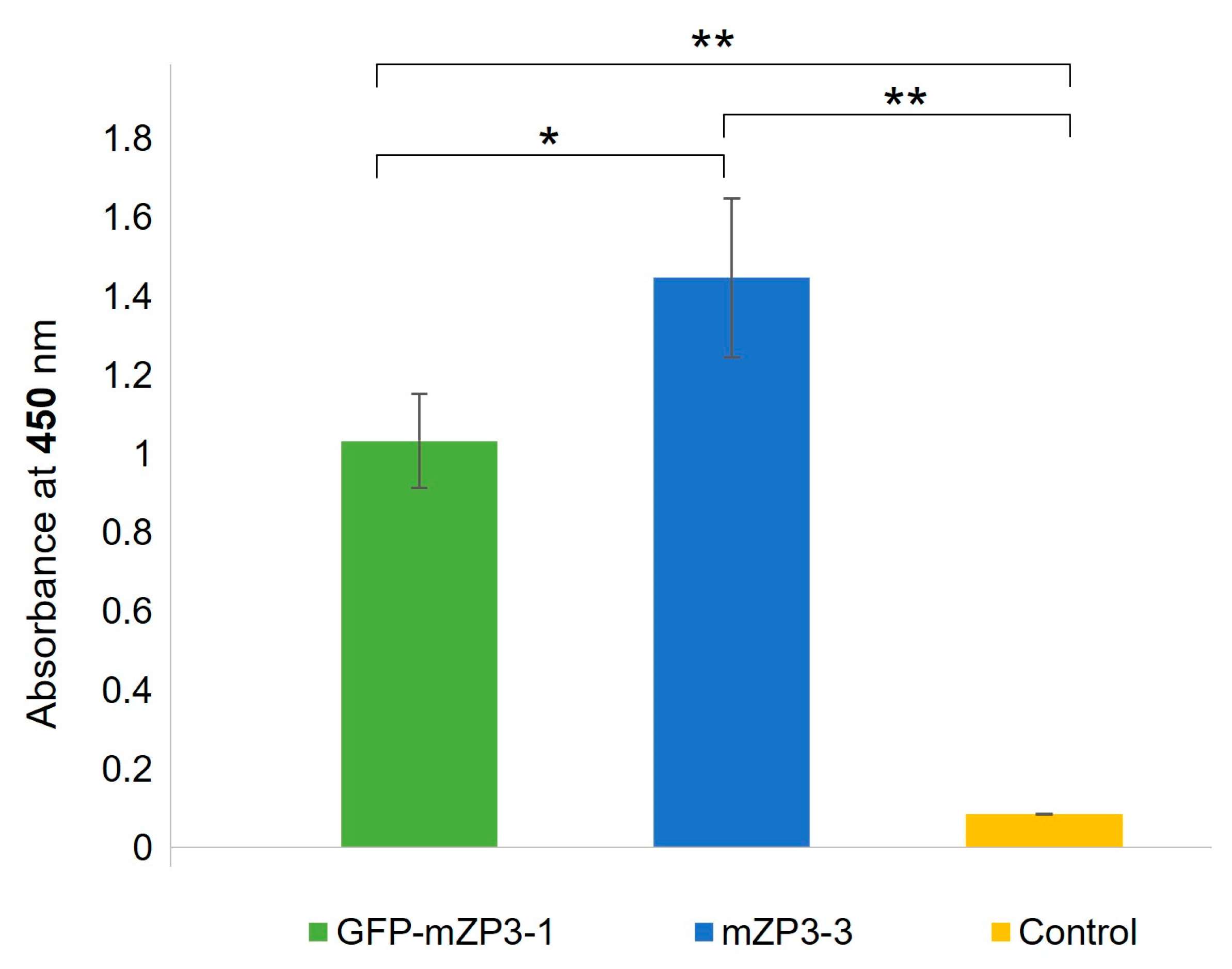
3.4. Immunogenicity of mZP3-Based Antigens
3.5. Fertility and Ovarian Effects in BALB/c Mice Immunized with mZP3 Antigens
3.6. Reactivity of Serum Antibodies with the Mouse ZP Matrix by Indirect Immunofluorescence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenseth, N.C.; Leirs, H.; Skonhoft, A.; Davis, S.A.; Pech, R.P.; Andreassen, H.P.; Singleton, G.R.; Lima, M.; Machang’u, R.S.; Makundi, R.H.; et al. Mice, rats, and people: The bio-economics of agricultural rodent pests. Front. Ecol. Environ. 2003, 1, 367–375. [Google Scholar] [CrossRef]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kaboodvandpour, S.; Leung, L.K.-P. Managing crop damage caused by house mice (Mus domesticus) in Australia. Integr. Zool. 2010, 5, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Wondifraw, B.T.; Tamene, M.Y.; Simegn, A.B. Assessment of crop damage by rodent pests from experimental barley crop fields in Farta District, South Gondar, Ethiopia. PLoS ONE 2021, 16, e0255372. [Google Scholar] [CrossRef]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef]
- Brown, P.R.; Henry, S. Impacts of House Mice on Sustainable Fodder Storage in Australia. Agronomy 2022, 12, 254. [Google Scholar] [CrossRef]
- Singleton, G.R.; Brown, P.R.; Jacob, J.; Aplin, K.P. Unwanted and unintended effects of culling: A case for ecologically-based rodent management. Integr. Zool. 2007, 2, 247–259. [Google Scholar] [CrossRef]
- Tran, T.T.; Hinds, L.A. Fertility control of rodent pests: A review of the inhibitory effects of plant extracts on ovarian function. Pest Manag. Sci. 2013, 69, 342–354. [Google Scholar] [CrossRef]
- Chambers, L.K.; Singleton, G.R.; Hood, G.M. Immunocontraception as a potential control method of wild rodent populations. Belg. J. Zool. 1997, 127, 145–156. [Google Scholar]
- Jackson, R.J. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol. Reprod. 1998, 58, 152–159. [Google Scholar] [CrossRef]
- Redwood, A.J.; Smith, L.M.; Lloyd, M.L.; Hinds, L.A.; Hardy, C.M.; Shellam, G.R. Prospects for virally vectored immunocontraception in the control of wild house mice (Mus domesticus). Wildl. Res. 2007, 34, 530. [Google Scholar] [CrossRef]
- Paterson, M.; Jennings, Z.A.; van Duin, M.; Aitken, R.J. Immunocontraception with Zona pellucida Proteins. Cells Tissues Organs 2000, 166, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.; Fayrer-Hosken, R.A. Possible mechanisms of mammalian immunocontraception. J. Reprod. Immunol. 2000, 46, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. Immunocontraceptive effect of Izumo and enhancement by combination vaccination. Mol. Reprod. Dev. 2008, 75, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Kakkar, V.; Suman, P.; Chakrabarti, K.; Vrati, S.; Gupta, S.K. Immunogenicity of zona pellucida glycoprotein-3 and spermatozoa YLP12 peptides presented on Johnson grass mosaic virus-like particles. Vaccine 2009, 27, 2948–2953. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Xie, N. Male infertility-related molecules involved in sperm-oocyte fusion. J. Reprod. Dev. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Miller, L.A.; Johns, B.E.; Killian, G.J. Immunocontraception of white-tailed deer using native and recombinant zona pellucida vaccines. Anim. Reprod. Sci. 2000, 63, 187–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.K.; Gupta, N.; Suman, P.; Choudhury, S.; Prakash, K.; Gupta, T.; Sriraman, R.; Nagendrakumar, S.B.; Srinivasan, V.A. Zona pellucida-based contraceptive vaccines for human and animal utility. J. Reprod. Immunol. 2011, 88, 240–246. [Google Scholar] [CrossRef]
- Garside, D.; Gebril, A.; Alsaadi, M.; Ferro, V.A. Fertility control in wildlife: Review of current status, including novel and future technologies. Adv. Exp. Med. Biol. 2014, 753, 467–488. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Ogita, K.; Ferro, V.A.; Kumasawa, K.; Tsutsui, T.; Kimura, T. Immunisation with a plasmid DNA vaccine encoding gonadotrophin releasing hormone (GnRH-I) and T-helper epitopes in saline suppresses rodent fertility. Vaccine 2008, 26, 1365–1374. [Google Scholar] [CrossRef]
- Samoylov, A.; Napier, I.; Morrison, N.; Cochran, A.; Schemera, B.; Wright, J.; Cattley, R.; Samoylova, T. DNA Vaccine Targeting Gonadotropin-Releasing Hormone Receptor and Its Application in Animal Contraception. Mol. Biotechnol. 2019, 61, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. Recent advances in contraceptive vaccine development: A mini-review. Hum. Reprod. 2005, 20, 3271–3283. [Google Scholar] [CrossRef] [PubMed]
- Bechert, U.; Bartell, J.; Kutzler, M.; Menino, A.; Bildfell, R.; Anderson, M.; Fraker, M. Effects of two porcine zona pellucida immunocontraceptive vaccines on ovarian activity in horses. J. Wild. Manag. 2013, 77, 1386–1400. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Structure and function of the zona pellucida: Identification and characterization of the proteins of the mouse oocyte’s zona pellucida. Dev. Biol. 1980, 76, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Skinner, S.; Carino, C.; Wang, N.; Cartwright, J. Structure and Function of the Proteins of the Mammalian Zona pellucida. Cells Tissues Organs 2000, 166, 148–164. [Google Scholar] [CrossRef]
- Avella, M.A.; Xiong, B.; Dean, J. The molecular basis of gamete recognition in mice and humans. Mol. Hum. Reprod. 2013, 19, 279–289. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, N.; Choudhury, S.; Rath, A.; Sivapurapu, N.; Gahlay, G.; Batra, D. Update on zona pellucida glycoproteins based contraceptive vaccine. J. Reprod. Immunol. 2004, 62, 79–89. [Google Scholar] [CrossRef]
- Hardy, C.M.; Hinds, L.A.; Kerr, P.J.; Lloyd, M.L.; Redwood, A.J.; Shellam, G.R.; Strive, T. Biological control of vertebrate pests using virally vectored immunocontraception. J. Reprod. Immunol. 2006, 71, 102–111. [Google Scholar] [CrossRef]
- Naz, R.K.; Saver, A.E. Immunocontraception for Animals: Current Status and Future Perspective. Am. J. Reprod. Immunol. 2016, 75, 426–439. [Google Scholar] [CrossRef]
- Pitcovski, J.; Gruzdev, N.; Abzach, A.; Katz, C.; Ben-Adiva, R.; Brand-Shwartz, M.; Yadid, I.; Ratzon-Ashkenazi, E.; Emquies, K.; Israeli, H.; et al. Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. Vaccine 2022, 40, 1098–1107. [Google Scholar] [CrossRef]
- Sun, W. A Contraceptive Peptide Vaccine Targeting Sulfated Glycoprotein ZP2 of the Mouse Zona Pellucida. Biol. Reprod. 1999, 60, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.M.; Clydesdale, G.; Mobbs, K.J. Development of mouse-specific contraceptive vaccines: Infertility in mice immunized with peptide and polyepitope antigens. Reproduction 2004, 128, 395–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardy, C.M.; Beaton, S.; Hinds, L.A. Immunocontraception in mice using repeated, multi-antigen peptides: Immunization with purified recombinant antigens. Mol. Reprod. Dev. 2008, 75, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, E.; Oehninger, S.; Schill, W.B.; Hinsch, K.D. Species specificity of human and murine anti-ZP3 synthetic peptide antisera and use of the antibodies for localization and identification of ZP3 or ZPC domains of functional significance. Hum. Reprod. 1999, 14, 419–428. [Google Scholar] [CrossRef][Green Version]
- Hardy, C.M.; Pekin, J.; ten Have, J. Mouse-specific immunocontraceptive polyepitope vaccines. Reprod. Suppl. 2002, 60, 19–30. [Google Scholar] [CrossRef]
- Hasegawa, A.; Hamada, Y.; Shigeta, M.; Koyama, K. Contraceptive potential of synthetic peptides of zona pellucida protein (ZPA). J. Reprod. Immunol. 2002, 53, 91–98. [Google Scholar] [CrossRef]
- Ringuette, M.J.; Chamberlin, M.E.; Baur, A.W.; Sobieski, D.A.; Dean, J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev. Biol. 1988, 127, 287–295. [Google Scholar] [CrossRef]
- Kinloch, R.A.; Sakai, Y.; Wassarman, P.M. Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M.; Jovine, L.; Litscher, E.S. Mouse zona pellucida genes and glycoproteins. Cytogenet. Genome Res. 2004, 105, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Litscher, E.S.; Wassarman, P.M. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc. Natl. Acad. Sci. USA 1998, 95, 6193–6197. [Google Scholar] [CrossRef]
- Millar, S.E.; Chamow, S.M.; Baur, A.W.; Oliver, C.; Robey, F.; Dean, J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 1989, 246, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Ang, J.; Thai, H.; McElveen, F.; Tung, K.S. A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J. Immunol. 1995, 155, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.M.; ten Have, J.F.; Mobbs, K.J.; Hinds, L.A. Assessment of the immunocontraceptive effect of a zona pellucida 3 peptide antigen in wild mice. Reprod. Fertil. Dev. 2002, 14, 151–155. [Google Scholar] [CrossRef] [PubMed]
- East, I.J.; Gulyas, B.J.; Dean, J. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: Effects on fertilization and early development. Dev. Biol. 1985, 109, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ferro, V.A.; Mordini, E. Peptide vaccines in immunocontraception. Curr. Opin. Mol. Ther. 2004, 6, 83–89. [Google Scholar]
- Kaumaya, P.T.; Kobs-Conrad, S.; Seo, Y.H.; Lee, H.; VanBuskirk, A.M.; Feng, N.; Sheridan, J.F.; Stevens, V. Peptide vaccines incorporating a ‘promiscuous’ T-cell epitope bypass certain haplotype restricted immune responses and provide broad spectrum immunogenicity. J. Mol. Recognit. 1993, 6, 81–94. [Google Scholar] [CrossRef]
- Pinkhasov, J.; Alvarez, M.L.; Rigano, M.M.; Piensook, K.; Larios, D.; Pabst, M.; Grass, J.; Mukherjee, P.; Gendler, S.J.; Walmsley, A.M.; et al. Recombinant plant-expressed tumour-associated MUC1 peptide is immunogenic and capable of breaking tolerance in MUC1.Tg mice. Plant Biotechnol. J. 2011, 9, 991–1001. [Google Scholar] [CrossRef]
- Fraser, C.C.; Altreuter, D.H.; Ilyinskii, P.; Pittet, L.; Lamothe, R.A.; Keegan, M.; Johnston, L.; Kishimoto, T.K. Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine 2014, 32, 2896–2903. [Google Scholar] [CrossRef]
- Arukha, A.P.; Minhas, V.; Shrestha, A.; Gupta, S.K. Contraceptive efficacy of recombinant fusion protein comprising zona pellucida glycoprotein-3 fragment and gonadotropin releasing hormone. J. Reprod. Immunol. 2016, 114, 18–26. [Google Scholar] [CrossRef]
- Redwood, A.J.; Harvey, N.L.; Lloyd, M.; Lawson, M.A.; Hardy, C.M.; Shellam, G.R. Viral vectored immunocontraception: Screening of multiple fertility antigens using murine cytomegalovirus as a vaccine vector. Vaccine 2007, 25, 698–708. [Google Scholar] [CrossRef]
- Streatfield, S.J.; Howard, J.A. Plant-based vaccines. Int. J. Parasitol. 2003, 33, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Streatfield, S.J.; Rybicki, E.P. Advances in molecular farming: Key technologies, scaled up production and lead targets. Plant Biotechnol. J. 2015, 13, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Gomord, V.; Faye, L. Posttranslational modification of therapeutic proteins in plants. Curr. Opin. Plant Biol. 2004, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.M. “Molecular Farming” in Plants: Achievements and Prospects. Biotechnol. Biotechnol. Equip. 1995, 9, 3–9. [Google Scholar] [CrossRef][Green Version]
- Ma, J.K.-C.; Drake, P.M.W.; Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Benchabane, M.; Goulet, C.; Rivard, D.; Faye, L.; Gomord, V.; Michaud, D. Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol. J. 2008, 6, 633–648. [Google Scholar] [CrossRef]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef]
- Marillonnet, S. In planta engineering of viral RNA replicons: Efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef]
- Gleba, Y.Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Kandzia, R.; Marillonnet, S.; El-Shemy, H.A. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [PubMed]
- Nausch, H.; Broer, I. Cyanophycinase CphE from P. alcaligenes produced in different compartments of N. benthamiana degrades high amounts of cyanophycin in plant extracts. Appl. Microbiol. Biotechnol. 2017, 101, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Sabalza, M.; Christou, P.; Capell, T. Recombinant plant-derived pharmaceutical proteins: Current technical and economic bottlenecks. Biotechnol. Lett. 2014, 36, 2367–2379. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, H.; Hurtado, J.; Stahnke, J.; Leuzinger, K.; Dent, M. Agroinfiltration as an Effective and Scalable Strategy of Gene Delivery for Production of Pharmaceutical Proteins. Adv. Tech. Biol. Med. 2013, 1, 103. [Google Scholar] [CrossRef]
- Faye, L.; Boulaflous, A.; Benchabane, M.; Gomord, V.; Michaud, D. Protein modifications in the plant secretory pathway: Current status and practical implications in molecular pharming. Vaccine 2005, 23, 1770–1778. [Google Scholar] [CrossRef]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of protein pharmaceuticals: An update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef]
- Daniell, H.; Singh, N.D.; Mason, H.; Streatfield, S.J. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009, 14, 669–679. [Google Scholar] [CrossRef]
- Hsieh, J.M.; Besserer, G.M.; Madej, M.G.; Bui, H.-Q.; Kwon, S.; Abramson, J. Bridging the gap: A GFP-based strategy for overexpression and purification of membrane proteins with intra and extracellular C-termini. Protein Sci. 2010, 19, 868–880. [Google Scholar] [CrossRef]
- Janczak, M.; Bukowski, M.; Górecki, A.; Dubin, G.; Dubin, A.; Wladyka, B. A systematic investigation of the stability of green fluorescent protein fusion proteins. Acta Biochim. Pol. 2015, 62, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ponndorf, D.; Ehmke, S.; Walliser, B.; Thoss, K.; Unger, C.; Görs, S.; Daş, G.; Metges, C.C.; Broer, I.; Nausch, H. Stable production of cyanophycinase in Nicotiana benthamiana and its functionality to hydrolyse cyanophycin in the murine intestine. Plant Biotechnol. J. 2017, 15, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Mason, H.S. Modifying the Replication of Geminiviral Vectors Reduces Cell Death and Enhances Expression of Biopharmaceutical Proteins in Nicotiana benthamiana Leaves. Front. Plant Sci. 2018, 9, 1974. [Google Scholar] [CrossRef] [PubMed]
- Hamorsky, K.T.; Kouokam, J.C.; Jurkiewicz, J.M.; Nelson, B.; Moore, L.J.; Husk, A.S.; Kajiura, H.; Fujiyama, K.; Matoba, N. N-glycosylation of cholera toxin B subunit in Nicotiana benthamiana: Impacts on host stress response, production yield and vaccine potential. Sci. Rep. 2015, 5, 8003. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Maldonado-Agurto, R.; Dickson, A.J. The endoplasmic reticulum and unfolded protein response in the control of mammalian recombinant protein production. Biotechnol. Lett. 2014, 36, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Margolin, E.; Oh, Y.J.; Verbeek, M.; Naude, J.; Ponndorf, D.; Meshcheriakova, Y.A.; Peyret, H.; van Diepen, M.T.; Chapman, R.; Meyers, A.E.; et al. Co-expression of human calreticulin significantly improves the production of HIV gp140 and other viral glycoproteins in plants. Plant Biotechnol. J. 2020, 18, 2109–2117. [Google Scholar] [CrossRef]
- Sadler, K.; Bird, P.H.; Brown, L.E.; Jackson, D.C. The antigenic and immunogenic properties of synthetic peptide immunocontraceptive vaccine candidates based on gamete antigens. Vaccine 2000, 18, 416–425. [Google Scholar] [CrossRef]
- Jinshu, X.; Jingjing, L.; Duan, P.; Zheng, Z.; Ding, M.; Jie, W.; Rongyue, C.; Zhuoyi, H. The immunogenicity of recombinant and dimeric gonadotrophin-releasing hormone vaccines incorporating a T-helper epitope and GnRH or repeated GnRH units. J. Immunol. Methods 2004, 289, 111–122. [Google Scholar] [CrossRef]
- Haigh, O.; Guo, H.; Edgtton, K.; Mather, M.; Herd, K.A.; Tindle, R.W. Multiple copies of a tumor epitope in a recombinant hepatitis B surface antigen (HBsAg) vaccine enhance CTL responses, but not tumor protection. Virology 2007, 368, 363–375. [Google Scholar] [CrossRef]
- Sandam, N.P.; Prakash, D.; Thimmareddy, P. Immunocontraceptive potential of a GnRH receptor-based fusion recombinant protein. J. Genet. Eng. Biotechnol. 2021, 19, 63. [Google Scholar] [CrossRef]
- Hardy, C.M.; ten Have, J.F.; Beaton, S.; Jackson, R.J.; Clydesdale, G. Contraceptive responses of mice immunized with purified recombinant mouse zona pellucida subunit 3 (mZP3) proteins. Reproduction 2003, 126, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bosch, D.; Schots, A. Plant glycans: Friend or foe in vaccine development? Expert Rev. Vaccines 2010, 9, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Wadhwa, N.; Gupta, S.K. Evaluation of recombinant fusion protein comprising dog zona pellucida glycoprotein-3 and Izumo and individual fragments as immunogens for contraception. Vaccine 2014, 32, 564–571. [Google Scholar] [CrossRef] [PubMed]

| Antigen | Time Point | mZP3 Peptide/TSP (%) | Purified mZP3 (µg/g LDW) |
|---|---|---|---|
| mZP3-1 | 4 dpi | 0.047 ± 0.006 | 3.4 ± 0.8 |
| GFP-mZP3-1 | 8 dpi | 0.95 ± 0.05 | 53.4 ± 1 |
| mZP3-3 | 6 dpi | 1.18 ± 0.12 | 32.7 ± 2.3 |
| Antigen | Total Mice | Number of Injection | Number of Fertile Mice | Litter Size (Mean ± SEM) | p |
|---|---|---|---|---|---|
| GFP-mZP3-1 | 5 | 3 | 5/5 | 5.7 ± 0.6 | N/S |
| mZP3-3 | 5 | 3 | 5/5 | 7 ± 1.5 | N/S |
| PBS | 5 | 3 | 5/5 | 7 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemian, K.; Broer, I.; Schön, J.; Kolp, N.; Killisch, R.; Huckauf, J. Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice. Vaccines 2023, 11, 153. https://doi.org/10.3390/vaccines11010153
Ghasemian K, Broer I, Schön J, Kolp N, Killisch R, Huckauf J. Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice. Vaccines. 2023; 11(1):153. https://doi.org/10.3390/vaccines11010153
Chicago/Turabian StyleGhasemian, Khadijeh, Inge Broer, Jennifer Schön, Nadine Kolp, Richard Killisch, and Jana Huckauf. 2023. "Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice" Vaccines 11, no. 1: 153. https://doi.org/10.3390/vaccines11010153
APA StyleGhasemian, K., Broer, I., Schön, J., Kolp, N., Killisch, R., & Huckauf, J. (2023). Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice. Vaccines, 11(1), 153. https://doi.org/10.3390/vaccines11010153






