Analysis of Adverse Effects of COVID-19 Vaccines Experienced by Healthcare Workers at Guizhou Provincial Staff Hospital, China
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection Procedure
2.3. Inclusion Criteria
2.4. Exclusion Criteria
- (1)
- Not an HCW;
- (2)
- People with a history of COVID-19 infection or vaccine allergies;
- (3)
- Women who were pregnant or breastfeeding;
- (4)
- People with severe chronic or immunocompromised diseases;
- (5)
- People aged < 18 years or >60 years.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, W.; Lee, L.A.; Liu, Y.; Scherpbier, R.W.; Wen, N.; Zhang, G.; Zhu, X.; Ning, G.; Wang, F.; Li, Y.; et al. Vaccine-preventable disease control in the People’s Republic of China: 1949–2016. Vaccine 2018, 36, 8131–8137. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado- Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Matthijsse, S.M.; Hontelez, J.A.; Naber, S.K.; Rozemeijer, K.; De Kok, I.M.; Bakker, R.; Van Ballegooijen, M.; Van Rosmalen, J.; De Vlas, S.J. Public health benefits of routine human papillomavirus vaccination for adults in the Netherlands: A mathematical modeling study. J. Infect. Dis. 2016, 214, 854–861. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, C.; An, J.; Song, Y.; Yu, P.; Li, J.; Gu, C.; Hu, D.; Jiang, Y.; Zhang, L.; et al. Development of recombinant COVID-19 vaccine based on CHO-produced, prefusion spike trimer and alum/CpG adjuvants. Vaccine 2021, 39, 7001–7011. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- WHO. Immunization Safety Surveillance: Guidelines for Immunization Programme Managers on Surveillance of Adverse Events Following Immunization. 3rd ed. Available online: https://www.who.int/publications/i/item/9789290617457 (accessed on 12 January 2016).
- WHO. Report of the Meeting on HPV Vaccine Coverage and Impact Monitoring. Available online: https://www.who.int/publications/i/item/WHO_IVB_10.05 (accessed on 20 November 2019).
- Influenza vaccine for 2020–2021. Med. Lett. Drugs Ther. 2020, 62, 145–150.
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.J.; Swift, P.A. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: An exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Guerrero, M.L.; Navarro, S.R.M.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef]
- Chen, M.; Yuan, Y.; Zhou, Y.; Deng, Z.; Feng, F.; Zou, H.; Sun, C. Safety of SARS-CoV-2 vaccines: A systematic review and meta-analysis of randomized controlled trials. Infect. Dis. Poverty 2021, 10, 94. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lim, S.Y.; Lee, J.-H.; Lim, J.S.; Kim, M.; Kwon, S.; Joo, J.; Kwak, S.H.; Kim, E.O.; Jung, J.; et al. Adverse Reactions of the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers in Korea. J. Korean Med. Sci. 2021, 36, e153. [Google Scholar] [CrossRef]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–15. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Nie, X.; Qian, L.; Sun, R.; Huang, B.; Dong, X.; Xiao, Q.; Zhang, Q.; Lu, T.; Yue, L.; Chen, S.; et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021, 184, 775–791.e14. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.Y.; Norris, C.M.; Oudit, G.Y. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef]
- Zhu, J.S.; Zhang, M.X.; Chien, M.C.; Yang, W.Y.; Shi, G.F.; Qui, S.; Tung, T.H.; Chen, H.X. Sex Differences in Adverse Reactions to an Inactivated SARS-CoV-2 Vaccine Among Medical Staff in China. Front. Med. 2021, 8, 731593. [Google Scholar] [CrossRef]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef]
- Maes, M.; Hendriks, D.; Van Gastel, A.; Demedts, P.; Wauters, A.; Neels, H.; Janca, A.; Scharpé, S. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology 1997, 22, 397–409. [Google Scholar] [CrossRef]
- Leonard, B.E. Stress and the Immune System: Immunological Aspects of Depressive Illness. Int. Rev. Psychiatry 2009, 2, 321–330. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Radio, C.N. A Multi-Ethnic Big Family. Available online: http://www.cnr.cn/2012zt/wangyankan/duocaigui/201206/t20120605_509809135.html (accessed on 4 May 2012).
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Kresch, E.; Achua, J.; Saltzman, R.; Khodamoradi, K.; Arora, H.; Ibrahim, E.; Kryvenko, O.N.; Almeida, V.W.; Firdaus, F.; Hare, J.M.; et al. COVID-19 Endothelial Dysfunction Can Cause Erectile Dysfunction: Histopathological, Immunohistochemical, and Ultrastructural Study of the Human Penis. World J. Men Health 2021, 39, 466–469. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- WHO. Vaccination: European Commission and World Health Organization Join Forces to Promote the Benefits of Vaccines. Available online: https://www.who.int/news/item/12-09-2019-vaccination-european-commission-and-world-health-organization-join-forces-to-promote-the-benefits-of-vaccines (accessed on 12 September 2019).
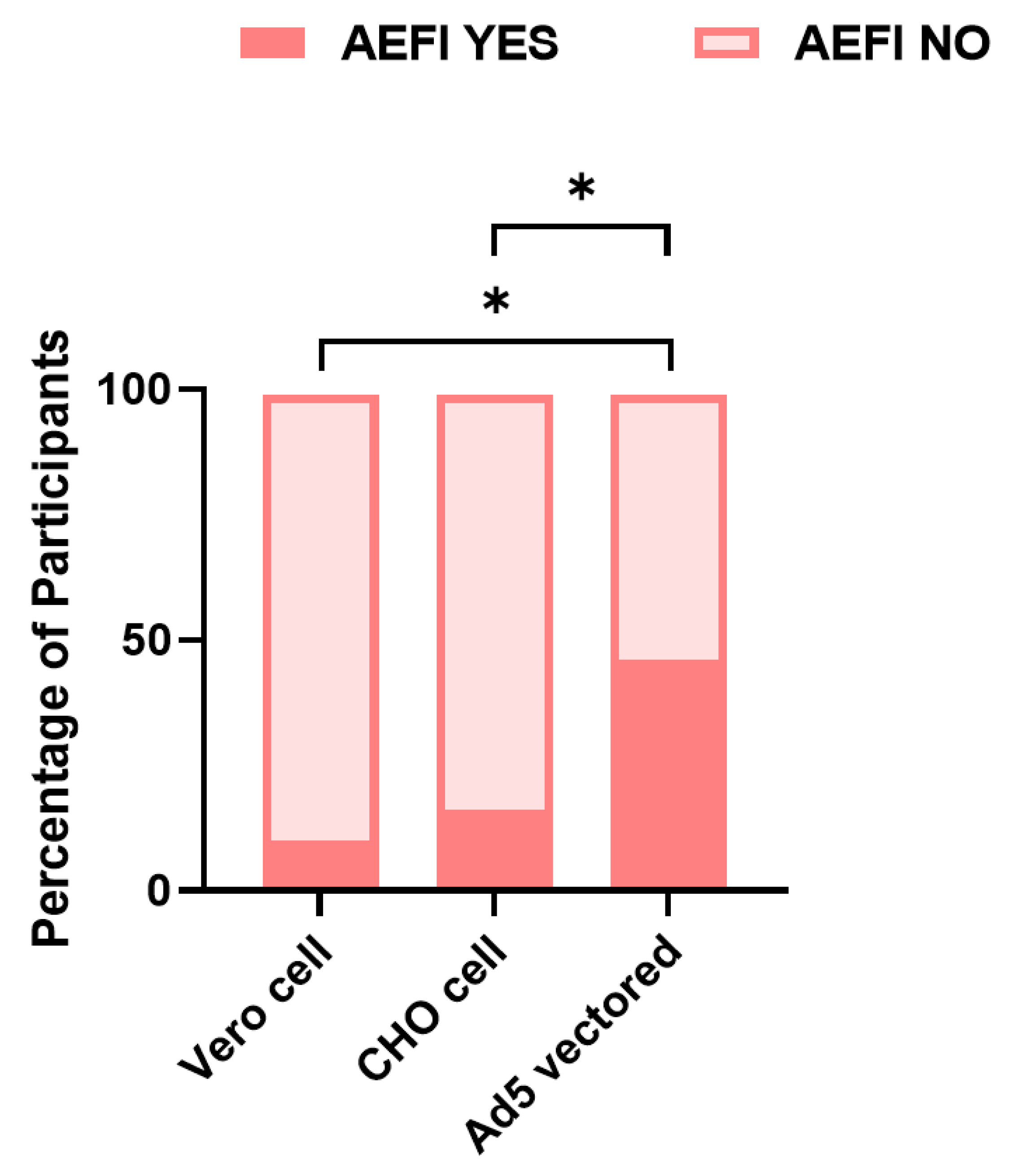
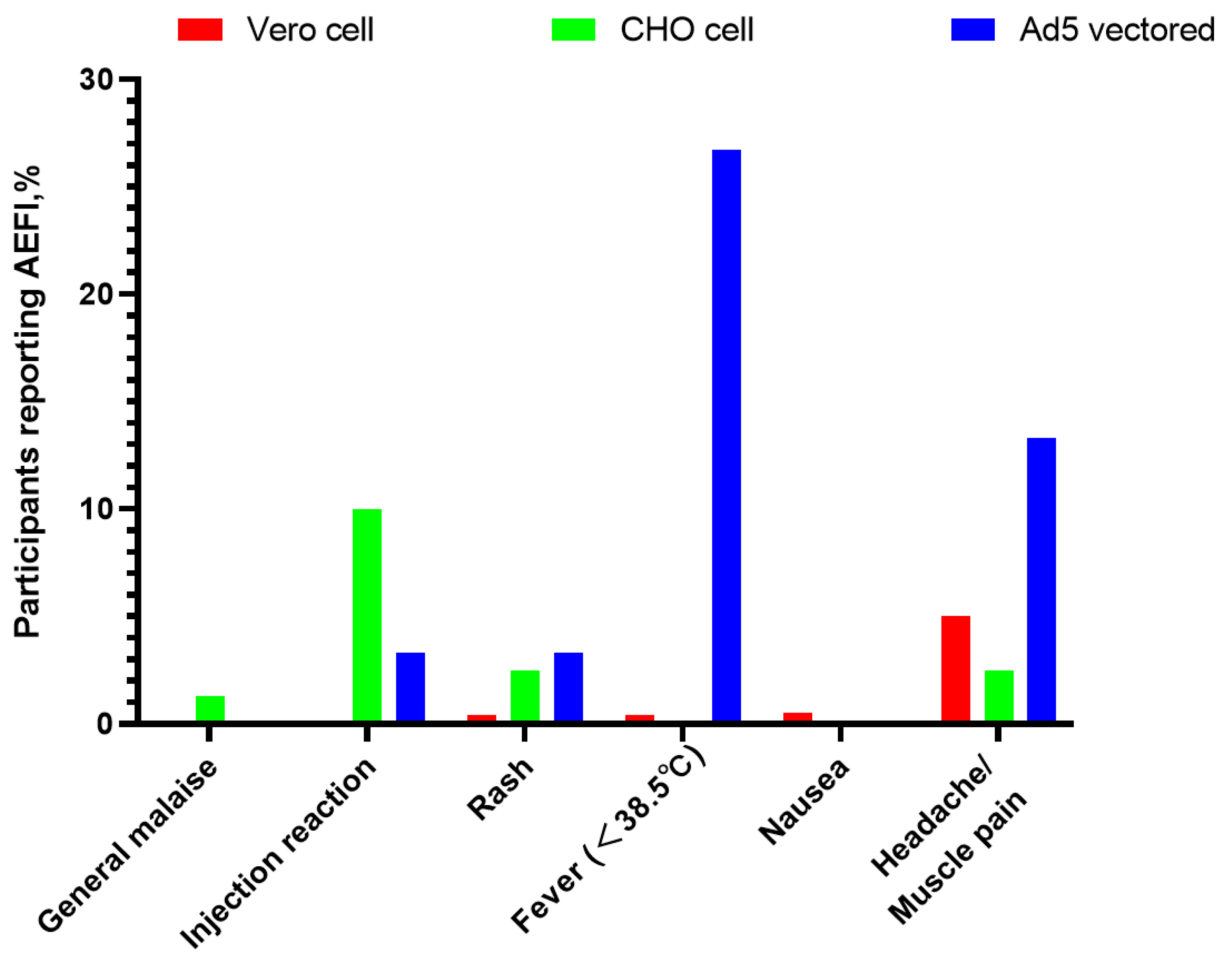
| Vero Cell Group | Cho Cell Group | Ad5 Vectored Group | Total | Statistic | p-Value | |
|---|---|---|---|---|---|---|
| Age group (in years) | ||||||
| 18–30 | 480 (60.5%) | 65 (81.3%) | 16 (53.3%) | 561 (62.1%) | χ2 = 14.8 | <0.022 |
| 31–40 | 166 (20.9%) | 7 (8.8%) | 8 (26.7%) | 181 (20%) | ||
| 41–50 | 76 (9.6%) | 5 (6.3%) | 3 (10%) | 84 (9.3%) | ||
| 51–60 | 72 (9.1%) | 3 (3.8%) | 3 (10%) | 78 (8.6) | ||
| Gender | χ2 = 21.9 | <0.001 | ||||
| Female | 562 (70.8%) | 73 (91.3%) | 28 (93.3%) | 663 (73.3%) | ||
| Male | 232 (29.2%) | 7 (8.8%) | 2 (6.7%) | 241 (26.7%) | ||
| Ethnicity | χ2 = 1.3 | 0.969 | ||||
| Han | 510 (64.2%) | 54 (67.5%) | 20 (66.7%) | 584 (64.6%) | ||
| Miao | 72 (9.1%) | 8 (10.0%) | 3 (10.0%) | 83 (9.2%) | ||
| Buyi | 58 (7.3) | 5 (6.3) | 1 (3.3%) | 64 (7.1%) | ||
| Others | 154 (19.4%) | 13 (16.3%) | 6 (19.1%) | 173 (19.1%) | ||
| Level of education | χ2 = 24.4 | <0.001 | ||||
| Bachelor’s degree or above | 416 (52.4%) | 19 (23.8%) | 17 (56.7%) | 452 (50%) | ||
| Junior college or blew | 378 (47.6%) | 61 (76.3%) | 13 (43.3%) | 452 (50%) |
| Vero Cell Group | Cho Cell Group | Ad5 Vectored Group | Total | Statistic | p Value | |
|---|---|---|---|---|---|---|
| Regression of Symptom | Fisher’s exact test | <0.001 | ||||
| Symptomless | 714 (90.3%) | 67 (83.8%) | 16 (53.3%) | 797 (88.2%) | ||
| Spontaneous remission | 80 (10.1%) | 11 (13.8%) | 12 (40.0%) | 103 (11.4%) | ||
| Seek help from outpatient provider | 0 | 2 (2.5%) | 2 (6.7%) | 4 (0.4) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Vero cell group | 0.128 (0.060–0.272) | <0.001 | 0.141 (0.065–0.306) | <0.001 |
| CHO cell group | 0.222 (0.087–0.563) | 0.002 | 0.279 (0.107–0.723) | 0.009 |
| Ad5 vectored group | Reference | Reference | ||
| Female | 2.428 (1.377–4.280) | 0.002 | 2.093 (1.171–3.742) | 0.013 |
| male | Reference | Reference | ||
| Bachelor’s degree or above | 2.055 (1.348–3.134) | 0.001 | 2.237 (1.434, 3.489) | <0.001 |
| Junior college or blew | Reference | Reference | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Wang, Y.; Liu, L.; Zha, Y.; Yang, Y.; Wang, Y.; Roberts, N.; Li, Y. Analysis of Adverse Effects of COVID-19 Vaccines Experienced by Healthcare Workers at Guizhou Provincial Staff Hospital, China. Vaccines 2022, 10, 1449. https://doi.org/10.3390/vaccines10091449
Wei Y, Wang Y, Liu L, Zha Y, Yang Y, Wang Y, Roberts N, Li Y. Analysis of Adverse Effects of COVID-19 Vaccines Experienced by Healthcare Workers at Guizhou Provincial Staff Hospital, China. Vaccines. 2022; 10(9):1449. https://doi.org/10.3390/vaccines10091449
Chicago/Turabian StyleWei, Yunhua, Yan Wang, Lin Liu, Yan Zha, Yuqi Yang, Yuanlin Wang, Neil Roberts, and Yaying Li. 2022. "Analysis of Adverse Effects of COVID-19 Vaccines Experienced by Healthcare Workers at Guizhou Provincial Staff Hospital, China" Vaccines 10, no. 9: 1449. https://doi.org/10.3390/vaccines10091449
APA StyleWei, Y., Wang, Y., Liu, L., Zha, Y., Yang, Y., Wang, Y., Roberts, N., & Li, Y. (2022). Analysis of Adverse Effects of COVID-19 Vaccines Experienced by Healthcare Workers at Guizhou Provincial Staff Hospital, China. Vaccines, 10(9), 1449. https://doi.org/10.3390/vaccines10091449





