Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review
Abstract
:1. Introduction
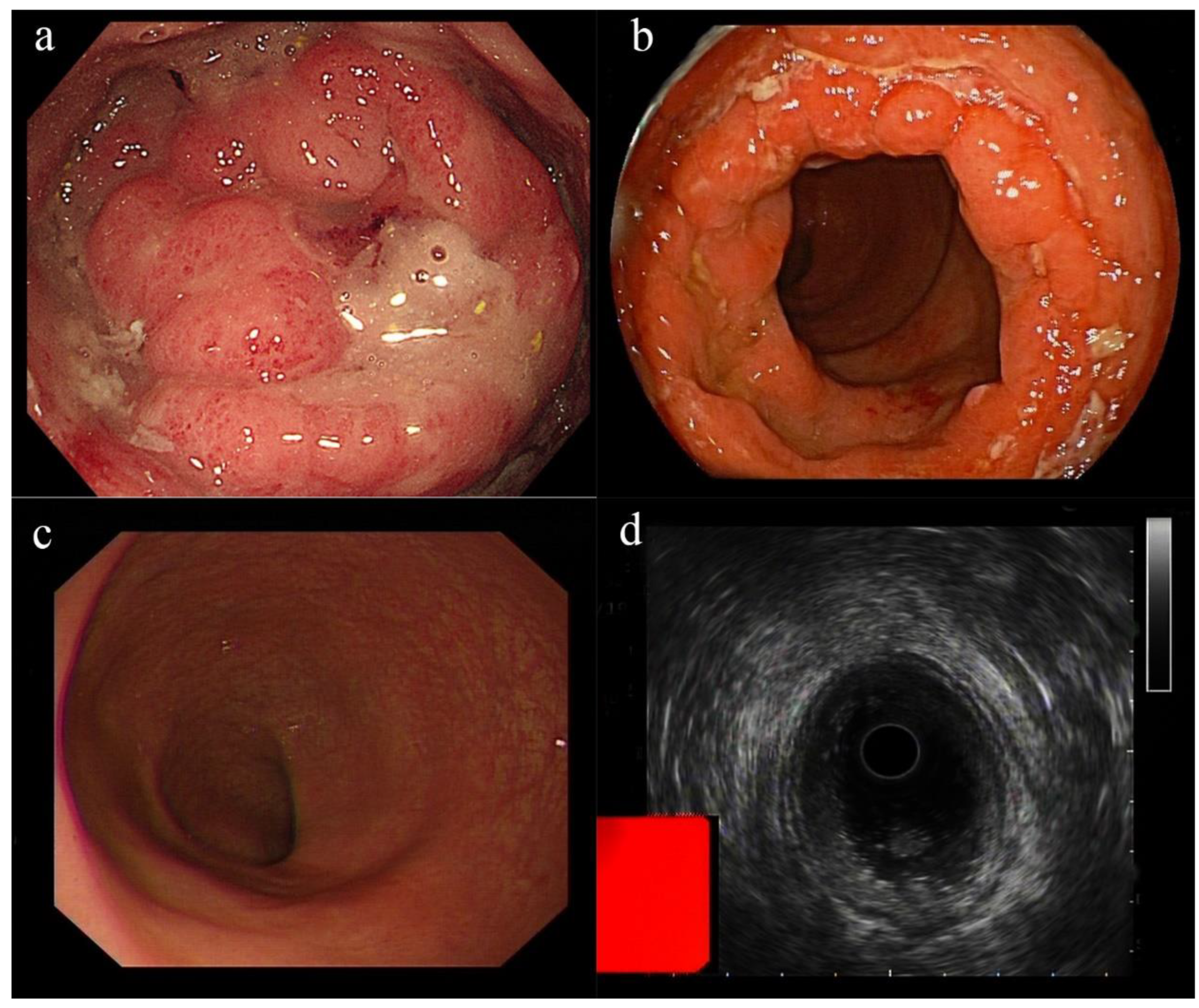
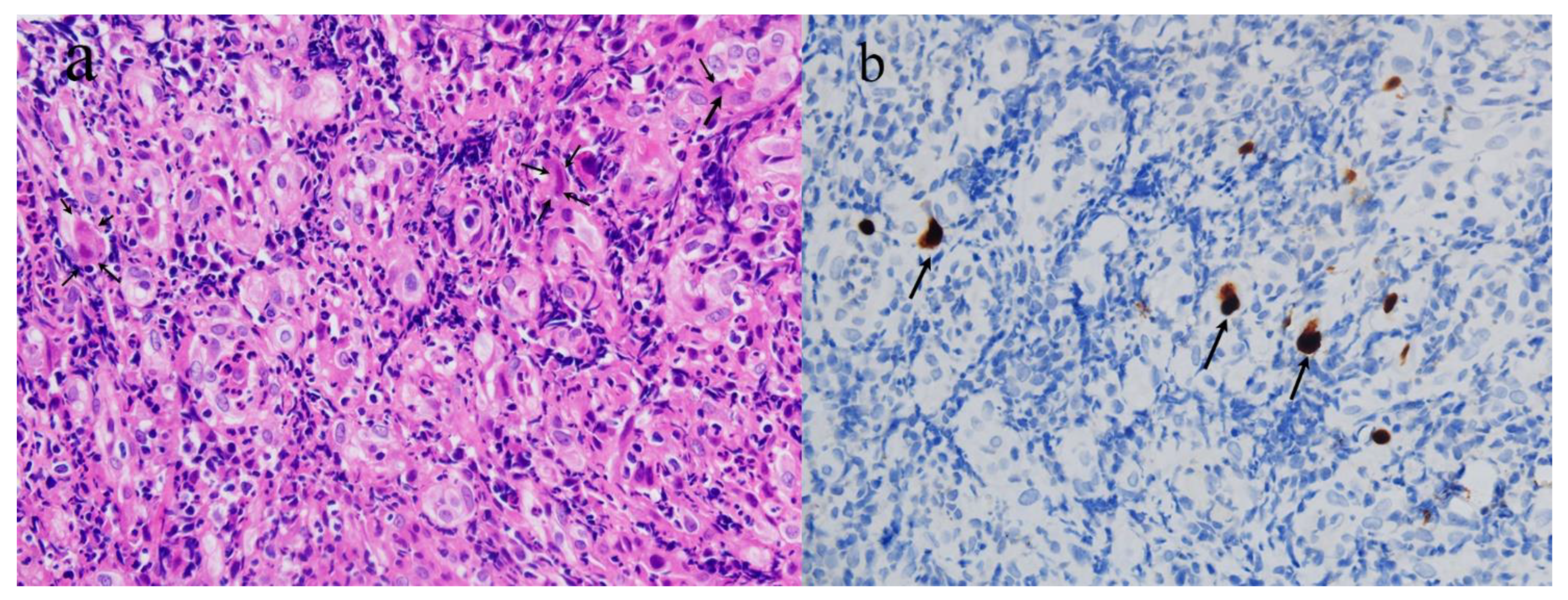
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H. Covid-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Eid, E.; Abdullah, L.; Kurban, M.; Abbas, O. Herpes zoster emergence following mRNA COVID-19 vaccine. J. Med. Virol. 2021, 93, 5231–5232. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Landriscina, A. Herpes Zoster Following COVID-19 Vaccination. J. Drugs Dermatol. 2021, 20, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.H.; Wei, K.C.; Chen, A.; Wang, W.H. Herpes zoster following COVID-19 vaccine: A report of three cases. Qjm 2021, 114, 531–532. [Google Scholar] [CrossRef]
- Maruki, T.; Ishikane, M.; Suzuki, T.; Ujiie, M.; Katano, H.; Ohmagari, N. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int. J. Infect. Dis. 2021, 113, 55–57. [Google Scholar] [CrossRef]
- Fathy, R.A.; McMahon, D.E.; Lee, C.; Chamberlin, G.C.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: A review of 40 cases in an International Dermatology Registry. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e6–e9. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef]
- Garrido, I.; Lopes, S.; Simões, M.S.; Liberal, R.; Lopes, J.; Carneiro, F.; Macedo, G. Autoimmune hepatitis after COVID-19 vaccine—more than a coincidence. J. Autoimmun. 2021, 125, 102741. [Google Scholar] [CrossRef]
- Bril, F.; Al Diffalha, S.; Dean, M.; Fettig, D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J. Hepatol. 2021, 75, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Cieślewicz, A.; Dudek, M.; Krela-Kaźmierczak, I.; Jabłecka, A.; Lesiak, M.; Korzeniowska, K. Pancreatic Injury after COVID-19 Vaccine-A Case Report. Vaccines 2021, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Sharko, A.; Farooqi, A.; Ying, G.W.; Sura, P. Acute Pancreatitis: A Possible Side Effect of COVID-19 Vaccine. Cureus 2021, 13, e14741. [Google Scholar] [CrossRef]
- Torrente, S.; Castiella, A.; Garmendia, M.; Zapata, E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol Hepatol. 2021, 45, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.; Shen, J.G.; Olazagasti, C.; Shams, S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021, 14, e242678. [Google Scholar] [CrossRef] [PubMed]
- Lensen, R.; Netea, M.G.; Rosendaal, F.R. Hepatitis C Virus Reactivation Following COVID-19 Vaccination—A Case Report. Int. Med. Case Rep. J. 2021, 14, 573–576. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef]
- Studemeister, A. Cytomegalovirus proctitis: A rare and disregarded sexually transmitted disease. Sex. Transm. Dis. 2011, 38, 876–878. [Google Scholar] [CrossRef]
- Ng, F.H.; Chau, T.N.; Cheung, T.C.; Kng, C.; Wong, S.Y.; Ng, W.F.; Lee, K.C.; Chan, E.; Lai, S.T.; Yuen, W.C. Cytomegalovirus colitis in individuals without apparent cause of immunodeficiency. Dig. Dis Sci. 1999, 44, 945–952. [Google Scholar] [CrossRef]
- Macaigne, G.; Auriault, M.L.; Boivin, J.F.; Chayette, C.; Cheaib, S.; Deplus, R. Acute cytomegalovirus (CMV) recto-colitis mimicking rectal carcinoma without apparent cause of immunodeficiency. Gastroenterol. Clin. Biol. 2004, 28, 73–76. [Google Scholar] [CrossRef]
- Subbarao, S.; O’Sullivan, A.; Adesina, T.; Gwozdz, A.M.; Rees, J.; Satta, G. Cytomegalovirus proctitis mimicking rectal cancer in an immunocompetent elderly patient: A case report. BMC Res. Notes 2014, 7, 799. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 Vaccine and Myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef]
- Fanni, D.; Saba, L.; Demontis, R.; Gerosa, C.; Chighine, A.; Nioi, M.; Suri, J.S.; Ravarino, A.; Cau, F.; Barcellona, D. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: A report of an autoptic case and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5063–5069. [Google Scholar]
- Yoshifuji, A.; Ishioka, K.; Masuzawa, Y.; Suda, S.; Murata, S.; Uwamino, Y.; Fujino, M.; Miyahara, H.; Hasegawa, N.; Ryuzaki, M. COVID-19 vaccine induced interstitial lung disease. J. Infect. Chemother. 2022, 28, 95–98. [Google Scholar] [CrossRef]
- Al-Mashdali, A.F.; Ata, Y.M.; Sadik, N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann. Med. Surg. 2021, 69, 102803. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, Y.H.; Lu, P.L. Reactivated cytomegalovirus proctitis in an immunocompetent patient presenting as nosocomial diarrhea: A case report and literature review. BMC Infect. Dis. 2017, 17, 113. [Google Scholar] [CrossRef]
- Plüß, M.; Mese, K.; Kowallick, J.T.; Schuster, A.; Tampe, D.; Tampe, B. Case Report: Cytomegalovirus Reactivation and Pericarditis Following ChAdOx1 nCoV-19 Vaccination Against SARS-CoV-2. Front. Immunol. 2021, 12, 784145. [Google Scholar] [CrossRef]
- Maillet, F.; Pourbaix, A.; le Pluart, D.; Sirmai, L.; Postolache, S.A.; Couvelard, A.; Houhou-Fidouh, N.; Males, L.; Deconinck, L.; Lescure, F. Cytomegalovirus proctitis as a complication of COVID-19 with immunosuppressive treatments. IDCases 2021, 24, e01111. [Google Scholar] [CrossRef]
- Geisen, W.R.; Berger, J.; Schwartz, C.; Reddy, A.; Rai, B.; Wadih, G.; Peck, J. Cytomegalovirus Enterocolitis secondary to experimental COVID-19 therapy. IDCases 2020, 22, e00962. [Google Scholar] [CrossRef] [PubMed]
- Marchi, G.; Vianello, A.; Crisafulli, E.; Maroccia, A.; Crinò, S.F.; Pecori, S.; Zamboni, G.A.; Mazzaferri, F.; Tacconelli, E.; Girelli, D. Cytomegalovirus-Induced Gastrointestinal Bleeding and Pancreatitis Complicating Severe Covid-19 Pneumonia: A Paradigmatic Case. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020060. [Google Scholar] [CrossRef] [PubMed]
| Author | Patient’s Age/Sex | Past Medical Condition | Diagnosis | The Interval between Vaccination and First Symptom | Presenting Symptoms | Confirmed Conditions | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Garrido et al. [10] | A 65-year-old woman | None | Autoimmune hepatitis | Two weeks after the first dose of Moderna COVID-19 vaccine | Mild abdominal pain, jaundice, and choluria | Liver histology showed a marked expansion of the portal tracts, severe interface hepatitis, and multiple confluent foci of lobular necrosis. Abdominal Doppler ultrasound showed hepatomegaly. Liver enzyme index increased | Treatment with prednisolone at 60 mg/day and a tapering course of corticosteroids. | Cure |
| Bril et al. [11] | A 35-year-old Caucasian female | Gestational hypertension | Autoimmune hepatitis | One 1 week after receiving her first dose of Pfizer–BioNTech COVID-19 vaccine | Generalized pruritus, choluria, and jaundice | Histology revealed the presence of eosinophils. Laboratories were significant for AST 754 U/L and ALT 2001 U/L. Doppler reported hepatomegaly without cirrhotic morphology | Treatment with prednisone at 20 mg daily | Cure |
| Cieślewicz et al. [12] | A 29-year-old female Caucasian | None | Pancreatic Injury | Twelve hours after the first dose of Pfizer–BioNTech COVID-19 mRNA vaccination | Muscle pain, headache, chills, and general weakness | Biochemical analysis revealed significantly increased CRP and urine amylase at 544 U/L. Magnetic resonance imaging of the abdomen suggested a mild pancreatic injury | The patient received paracetamol at 1 g i.v., a strict diet of fluids, gastroresistant capsules of pancreatic enzymes, and proton pump inhibitors | Cure |
| Parkash et al. [13] | A 96-year-old Caucasian female | Diastolic congestive heart failure, hypertension, hypothyroidism, cholecystectomy, and appendectomy | Acute pancreatitis | A few days after getting the first dose of Pfizer–BioNTech COVID-19 vaccine | Acute onset, severe abdominal pain | Her lipase level was significantly elevated, at 4036 U/L | She was monitored overnight with conservative treatment | Cure |
| Torrente et al. [14] | A 46-year-old Caucasian woman | Hypothyroidism and chronic iron deficiency anemia | Autoimmune hepatitis | 3 weeks after the first Vaxzevria COVID-19 vaccination | Asymptomatic | Hypertransaminasemia. Laboratories showed AST 241 U/L, ALT 353 U/L, and GGT 44 U/L. Liver biopsy showed lymphoplasmacytic portal infiltrate with focal disruption of the limiting plate | Prednisone was initiated at a dose of 30 mg daily with a rapid improvement after 2 weeks of treatment, and azathioprine was added to treatment at a dose of 50 mg daily. | Cure |
| Hines et al. [15] | A 26-year-old woman | Irregular menses on oral contraceptives | ITP and acute liver injury | 2 weeks after receiving the Moderna mRNA-1273 SARS-CoV-2 vaccine | Petechial rash | The peripheral blood smear showed rare schistocytes, and giant platelets, with her AST and ALT levels peaking on hospital day 3 at 446 U/L and 1257 U/L | Oral prednisone at 40 mg/day for 3 days. Dexamethasone at 40 mg IVP for 4 days. IVIG at 1 g/kg for 2 days. | Cure |
| Lensen et al. [1] | An 82-year-old woman | Alzheimer’s disease, HBV infection, HCV infection, DM, essential hypertension, osteoarthritis, portal hypertension with esophageal varices, and hepatic cirrhosis with thrombocytopenia | Hepatitis C virus reactivation | 3 days after COVID-19 using Pfizer–BioNTech COVID-19 vaccine (first dose) | Jaundice, loss of consciousness, hepatic coma, and death | Hepatitis C PCR and hepatitis C antibodies were positive | Patient refused treatment with hepatitis C medication | Dead |
| Eid, E. et al. [4] | A 79-year-old man | Hypertension, coronary artery disease, and antineutrophilic cytoplasmic antibody-related glomerulonephritis | Herpes zoster | 6 days after receiving the mRNA COVID-19 vaccine | Itchy and tender lesions over the right thigh | On dermatologic examination, a confluence of vesicles, some excoriated and overlying an erythematous base, were appreciated scattered over the right thigh in a dermatomal distribution | Systemic antiviral treatment | Cure |
| David, E. et al. [5] | A 41-year-old woman | A history of varicella infection in childhood | Herpes zoster | 3 days after vaccination with Moderna COVID-19 | Fatigue and left arm soreness around the injection site, diarrhea, skin pain affecting the left lower back, and vesicular rash | Physical examination revealed a cluster of pink to red erythematous urticarial appearing papules and plaques with overlying clustered vesicles. Vesicular fluid was collected for VZV DNA PCR, which yielded a positive result. | Without treatment | Cure |
| Chiu, H.H. et al. [6] | A 71-year-old man | None | Herpes zoster | 2 days after his first injection of Moderna COVID-19 vaccine | grouped erythematous papules and vesicles appeared on his left flank with itching and pain | Based on clinical manifestations, HZ involving left T8 dermatome was diagnosed | Oral acyclovir for 1 week | Cure |
| A 46-year-old man | None | Herpes zoster | 2 days following receiving his first dose of AZD1222 vaccine | Pain and itch over ipsilateral flank | HZ was diagnosed later when the typical clinical presentation of HZ as grouped vesicles was present over left T11 | Oral acyclovir for 1 week | Cure | |
| Kerr, C. et al. [7] | A 71-year-old woman | Immunoglobulin A nephritis, and a history of chickenpox in childhood | Varicella-zoster virus meningitis | 1 day after her first BNT162b2 mRNA COVID-19 vaccination | Fever and headache | A final diagnosis of VZV meningitis was made based on positive rapid immunochromatography (abdominal vesicles tested) and CSF polymerase chain reaction (PCR) results (1.86 × 106/μL) | Intravenous acyclovir treatment | Cure |
| Plüß, M. et al. [29] | A 67-year-old Caucasian female | Atrial fibrillation, hypertension, obesity, degenerative knee joint disease, and no documented history of COVID-19 | Cytomegalovirus reactivation and pericarditis | Two weeks after first dose of ChAdOx1 nCoV-19 vaccination | She suffered from fever, weakness, and arthralgia of the knees, hips, and shoulders | Cardiac magnetic resonance imaging (MRI) confirmed diagnosis of pericarditis with circumferential thickening and contrast enhancement of the entire pericardium at late gadolinium enhancement. CMV infection was confirmed by PCR with detectable CMV viremia | Oral valganciclovir was initiated (900 mg twice daily) for three weeks | Cure |
| Our report | A 58-year-old Chinese woman | None | CMV proctitis | Three days after the second dose of CoronaVac COVID-19 vaccine | Constipation, perianal discomfort, and abdominal distention | Proctosigmoidoscopy revealed new circumferential growth at the anorectal junction, with an uneven surface and ulceration. A biopsy revealed moderately active chronic proctitis with CMV infection | The patient received 250 mg of ganciclovir twice daily for 5 days, and oral ganciclovir was continued after discharge | Cure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Chang, Y. Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review. Vaccines 2022, 10, 1417. https://doi.org/10.3390/vaccines10091417
Lv Y, Chang Y. Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review. Vaccines. 2022; 10(9):1417. https://doi.org/10.3390/vaccines10091417
Chicago/Turabian StyleLv, Yuqing, and Ying Chang. 2022. "Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review" Vaccines 10, no. 9: 1417. https://doi.org/10.3390/vaccines10091417
APA StyleLv, Y., & Chang, Y. (2022). Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review. Vaccines, 10(9), 1417. https://doi.org/10.3390/vaccines10091417





