Acceptance of Booster COVID-19 Vaccine and Its Association with Components of Vaccination Readiness in the General Population: A Cross-Sectional Survey for Starting Booster Dose in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Participants and Data Collection
2.2. Measurement Method
2.2.1. Sociodemographic Factors, COVID-19 Incidence, and Adverse Events in Priming
2.2.2. COVID-19 Vaccine History and Intention to Be Vaccinated with a Booster Dose
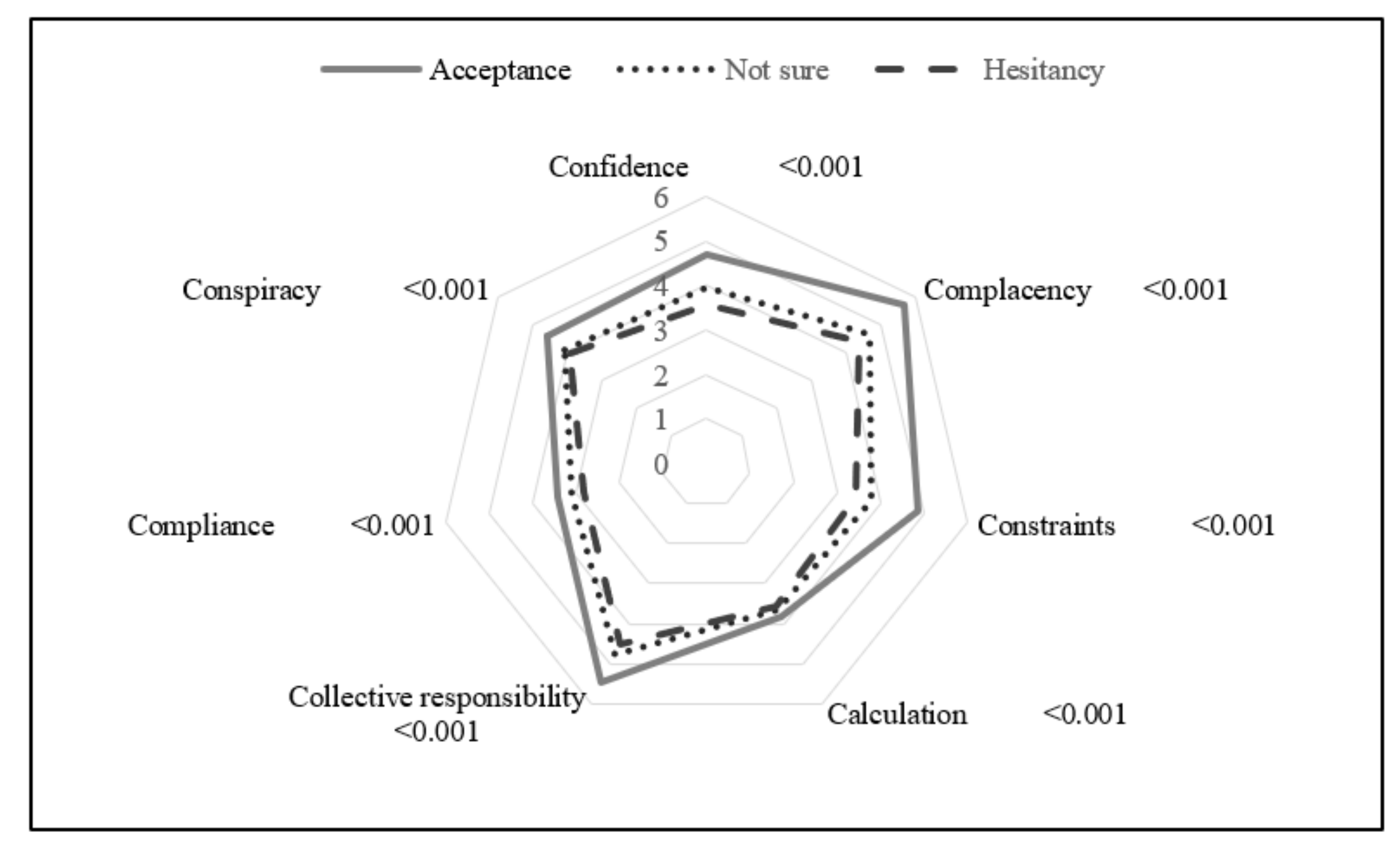
2.2.3. 7C Vaccination Readiness Scale
2.2.4. Vaccine Social Norms
2.3. Statistical Analyses
3. Results
4. Discussion
4.1. Booster Dose Vaccination Intention
4.2. Suitability of 7C Vaccination Readiness Scale for General Japanese Population
4.3. COVID-19 Vaccination Issue in the General Population
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NHK (Japan Broadcasting Corporation). Special Site: New Coronavirus. Vaccination Status in Japan. Available online: https://www3.nhk.or.jp/news/special/coronavirus/vaccine/progress/ (accessed on 3 February 2022).
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare. Notice Regarding Additional Vaccination (3rd Dose) COVID-19 Vaccines. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_booster.html (accessed on 3 February 2022).
- Tokiya, M.; Hara, M.; Matsumoto, A.; Ashenagar, M.S.; Nakano, T.; Hirota, Y. Association of vaccine confidence and hesitancy in three phases of COVID-19 vaccine approval and introduction in Japan. Vaccines 2022, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, M.; Gadoth, A.; Martin-Blais, R.; Gray, A.N.; Kashani, S.; Kazan, C.; Kane, B.; Tobin, N.H.; Ferbas, K.G.; Aldrovandi, G.M.; et al. Longitudinal assessment of coronavirus disease 2019 vaccine acceptance and uptake among frontline medical workers in Los Angeles, California. Clin. Infect. Dis. 2022, 74, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Folcarelli, L.; del Giudice, G.M.; Corea, F.; Angelillo, I.F. Intention to receive the COVID-19 vaccine booster dose in a university community in Italy. Vaccines 2022, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Yadete, T.; Batra, K.; Netski, D.M.; Antonio, S.; Patros, M.J.; Bester, J.C. Assessing acceptability of COVID-19 vaccine booster dose among adult Americans: A cross-sectional study. Vaccines 2021, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedziałek, B.; Fal, A. Willingness to receive the booster COVID-19 vaccine dose in Poland. Vaccines 2021, 9, 1286. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Fukushima, A.; Shimoda, K. Attitudes of medical students toward COVID-19 vaccination: Who is willing to receive a third dose of the vaccine? Vaccines 2021, 9, 1295. [Google Scholar] [CrossRef]
- NLI Research Institute. The 6th Survey on Changes in Life Due to COVID-19 (2020 2021 Nendo Tokubetu Chosa dai 6kai Shingata Korona Niyoru Kurashi no Henka ni Kansuru Chyosa Chosa Kekka Gaiyou). Available online: https://www.nli-research.co.jp/report/detail/id=69046?site=nli (accessed on 28 February 2022).
- Nikkei Inc. Japanese Inoculation Status Seen in the Chart Corona Vaccine. Available online: https://vdata.nikkei.com/newsgraphics/coronavirus-japan-vaccine-status/ (accessed on 25 February 2022).
- Agranov, M.; Elliott, M.; Ortoleva, P. The importance of social norms against strategic effects: The case of covid-19 vaccine uptake. Econ. Lett. 2021, 206, 109979. [Google Scholar] [CrossRef]
- Jaffe, A.E.; Graupensperger, S.; Blayney, J.A.; Duckworth, J.C.; Stappenbeck, C.A. The role of perceived social norms in college student vaccine hesitancy: Implications for COVID-19 prevention strategies. Vaccine 2022, 40, 1888–1895. [Google Scholar] [CrossRef]
- Hara, M.; Ishibashi, M.; Nakane, A.; Nakano, T.; Hirota, Y. Differences in COVID-19 vaccine acceptance, hesitancy, and confidence between healthcare workers and the general population in Japan. Vaccines 2021, 9, 1389. [Google Scholar] [CrossRef]
- Kajikawa, N.; Yokoya, S.; Maeno, T. Covid-19 vaccination willingness and associated factors in Japanese primary care patients: A cross-sectional study. J. Prim. Care Community Health 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Rees, F.; Lilleholt, L.; Santana, A.P.; Zettler, I.; Wilhelm, O.; Betsch, C.; Böhm, R. Measuring the 7C of vaccination readiness. Eur. J. Psychol. Assess. 2021, 1–9. [Google Scholar] [CrossRef]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef] [Green Version]
- Homepage: Vaccination-Readiness. Available online: https://www.vaccination-readiness.com/ (accessed on 25 February 2022).
- Machida, M.; Kojima, T.; Popiel, H.A.; Odagiri, Y.; Inoue, S. Japanese Version of the 7Cs of Vaccination Readiness. Available online: http://www.tmu-ph.ac/study/pandemic.php (accessed on 6 May 2022).
- World Health Organization. COVID-19 Advice for the Public: Getting Vaccinated. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 10 March 2022).
- Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 9 March 2022).
- Statista. The Countries where COVID-19 Vaccination is Mandatory. Available online: https://cdn.statcdn.com/Infographic/images/normal/25326.jpeg (accessed on 14 March 2022).
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of influenza vaccination intention and behavior-a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef]
- Hornsey, M.J.; Harris, E.A.; Fielding, K.S. The psychological roots of anti-vaccination attitudes: A 24-nation investigation. Health Psychol. 2018, 37, 307–315. [Google Scholar] [CrossRef]
- Sprengholz, P.; Felgendreff, L.; Böhm, R.; Betsch, C. Vaccination policy reactance: Predictors, consequences, and countermeasures. J. Health Psychol. 2022, 27, 1394–1407. [Google Scholar] [CrossRef]
- Okuhara, T.; Ishikawa, H.; Kato, M.; Okada, M.; Kiuchi, T. A qualitative analysis of the beliefs of Japanese anti-influenza vaccination website authors. Heliyon 2018, 4, e00609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Council of the Ministry of Health, Labour and Welfare (Immunization and Vaccine Working Group) (Kousei Kagaku Shingikai Yobou Sessyu Wakutin Bunkakai). The Approach to and Evaluation of Adverse Reactions to Vaccines (Wakuchin no Hukuhannou ni Taisuru Kangaekata Oyobi Hyouka ni Tuite). Available online: https://www.mhlw.go.jp/content/10601000/000739054.pdf (accessed on 25 March 2022).
- Habersaat, K.B.; Jackson, C. Understanding vaccine acceptance and demand-and ways to increase them. Bundesgesundheitsblatt Gesundh. Gesundh. 2020, 63, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- DiClemente, R.J.; Jackson, J.M. Risk communication. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Oxford Academic Press: Oxford, UK, 2017; pp. 378–382. [Google Scholar]
- Bahri, P.; Rägo, L. CIOMS guide to vaccine safety communication—Executive summary. Vaccine 2019, 37, 401–408. [Google Scholar] [CrossRef]
- Bahri, P.; Castillon Melero, M. Listen to the public and fulfil their information interests-translating vaccine communication research findings into guidance for regulators. Br. J. Clin. Pharmacol. 2018, 84, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccine Confidence Project. Vaccines and Global Health: The Week in Review. Available online: https://www.vaccineconfidence.org/ (accessed on 6 May 2022).
- Biswas, M.R.; Alzubaidi, M.S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z. A scoping review to find out worldwide COVID-19 vaccine hesitancy and its underlying determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Al-Amer, R.; Maneze, D.; Everett, B.; Montayre, J.; Villarosa, A.R.; Dwekat, E.; Salamonson, Y. COVID-19 vaccination intention in the first year of the pandemic: A systematic review. J. Clin. Nurs. 2022, 31, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Sabella, E.A.; Roma, P.; Ferracuti, S.; Da Molin, G.; Diella, G.; Montagna, M.T.; Orsi, G.B.; Liguori, G.; Napoli, C. Knowledge and lifestyle behaviors related to covid-19 pandemic in people over 65 years old from southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10872. [Google Scholar] [CrossRef]
| n | (%) | |
|---|---|---|
| Strongly agree | 2317 | 37.5 |
| Agree | 2515 | 40.8 |
| Neither or not | 925 | 15.0 |
| Disagree | 289 | 4.7 |
| Strongly disagree | 126 | 2.0 |
| n | Acceptance n = 4832 n (%) | Not Sure n = 925 n (%) | Hesitancy n = 415 n (%) | p-Value | ||
|---|---|---|---|---|---|---|
| Sex | Male | 2890 | 2319 (80.2) | 386 (13.4) | 185 (6.4) | 0.001 |
| Female | 3282 | 2513 (76.6) | 539 (16.4) | 230 (7.0) | ||
| Age | (Mean: years old) | 52.5 | 43.2 | 42.6 | <0.001 | |
| 20–29 | 791 | 521 (65.9) | 180 (22.8) | 90 (11.4) | <0.001 | |
| 30–39 | 1054 | 688 (65.3) | 254 (24.1) | 112 (10.6) | ||
| 40–49 | 1133 | 828 (73.1) | 211 (18.6) | 94 (8.3) | ||
| 50–59 | 1151 | 957 (83.2) | 137 (11.9) | 57 (5.0) | ||
| 60–69 | 1018 | 903 (88.7) | 81 (8.0) | 34 (3.3) | ||
| 70–79 | 1025 | 935 (91.2) | 62 (6.1) | 28 (2.7) | ||
| Area | Hokkaido | 291 | 217 (74.6) | 56 (19.2) | 18 (6.2) | 0.193 |
| Tohoku | 336 | 266 (79.2) | 40 (11.9) | 30 (8.9) | ||
| Kanto | 2373 | 1890 (79.7) | 334 (14.1) | 149 (6.3) | ||
| Chubu | 994 | 755 (76.0) | 164 (16.5) | 75 (7.6) | ||
| Kinki | 1211 | 945 (78.0) | 182 (15.0) | 84 (6.9) | ||
| Chugoku | 307 | 232 (75.6) | 56 (18.2) | 19 (6.2) | ||
| Shikoku | 145 | 117 (80.7) | 20 (13.8) | 8 (5.5) | ||
| Kyusyu | 515 | 410 (79.6) | 73 (14.2) | 32 (6.2) | ||
| Married | No | 2275 | 1680 (73.9) | 402 (17.7) | 193 (8.5) | <0.001 |
| Yes | 3897 | 3152 (80.9) | 523 (13.4) | 222 (5.7) | ||
| Child | No | 2507 | 1822 (72.7) | 470 (18.8) | 215 (8.6) | <0.0001 |
| Yes | 3665 | 3010 (82.1) | 455 (12.4) | 200 (5.5) | ||
| Annual household income | <JPY 4 million | 1654 | 1312 (79.3) | 234 (14.2) | 108 (6.5) | <0.001 |
| ≥JPY 4 million | 3170 | 2539 (80.1) | 440 (13.9) | 191 (6.0) | ||
| Unknown | 1348 | 981 (72.8) | 251 (18.6) | 116 (8.6) | ||
| Educational attainment | High school graduate | 1781 | 1404 (78.8) | 251 (14.1) | 126 (7.1) | 0.389 |
| Above higher education | 4391 | 3428 (78.1) | 674 (15.4) | 289 (6.6) | ||
| Body mass index ≥ 25 kg/m2 | No | 5013 | 3885 (77.5) | 773 (15.4) | 355 (7.1) | 0.005 |
| Yes | 1159 | 947 (81.7) | 152 (13.1) | 60 (5.2) | ||
| Underlying disease | No | 3965 | 2956 (74.6) | 698 (17.6) | 311 (7.8) | <0.001 |
| Yes | 2207 | 1876 (85.0) | 227 (10.3) | 104 (4.7) | ||
| Smoking | No | 5225 | 4075 (78.0) | 787 (15.1) | 363 (7.0) | 0.218 |
| Yes | 947 | 757 (79.9) | 138 (14.6) | 52 (5.5) | ||
| SARS-CoV-2 infection status (myself) | No | 6116 | 4790 (78.3) | 916 (15.0) | 410 (6.7) | 0.870 |
| Yes | 56 | 42 (75.0) | 9 (16.1) | 5 (8.9) | ||
| SARS-CoV-2 infection status (family, etc.) | No | 5396 | 4241 (78.6) | 811 (15.0) | 344 (6.4) | 0.016 |
| Yes | 776 | 591 (76.2) | 114 (14.7) | 71 (9.2) | ||
| SARS-CoV-2 infection status (inspection only) | No | 5344 | 4191 (78.4) | 807 (15.1) | 346 (6.5) | 0.127 |
| Yes | 828 | 641 (77.4) | 118 (14.3) | 69 (8.3) | ||
| If most people take a booster dose, I will do too (Social norms) | Strongly disagree | 151 | 67 (44.4) | 4 (2.7) | 80 (53.0) | <0.001 |
| Disagree | 326 | 133 (40.8) | 26 (8.0) | 167 (51.2) | ||
| Neither or not | 1159 | 481 (41.5) | 560 (48.3) | 118 (10.2) | ||
| Agree | 2766 | 2401 (86.8) | 319 (11.5) | 46 (1.7) | ||
| Strongly agree | 1770 | 1750 (98.9) | 16 (0.9) | 4 (0.2) |
| Adverse Events/Yes | Acceptance n = 4832 n (%) | Not Sure n = 925 n (%) | Hesitancy n = 415 n (%) | p-Value * |
|---|---|---|---|---|
| Lumps at the vaccination site | 870 (18.0) | 155 (16.8) | 58 (14.0) | 0.093 |
| Itching at the vaccination site | 626 (13.0) | 144 (15.6) | 64 (15.4) | 0.052 |
| Pain at the vaccination site | 3210 (66.4) | 632 (68.3) | 275 (66.3) | 0.525 |
| Redness at the vaccination site | 979 (20.3) | 198 (21.4) | 79 (19.0) | 0.577 |
| Swelling of the inoculation site | 1517 (31.4) | 299 (32.3) | 152 (36.6) | 0.086 |
| Fever | 2142 (44.3) | 517 (55.9) | 259 (62.4) | <0.001 |
| Tiredness, fatigue | 2039 (42.2) | 491 (53.1) | 234 (56.4) | <0.001 |
| Headache | 1036 (21.4) | 293 (31.7) | 157 (37.8) | <0.001 |
| Chills | 534 (11.1) | 167 (18.1) | 97 (23.4) | <0.001 |
| Vomiting | 64 (1.3) | 24 (2.6) | 17 (4.1) | <0.001 |
| Diarrhea | 80 (1.7) | 19 (2.1) | 24 (5.8) | <0.001 |
| Muscular pain | 1092 (22.6) | 266 (28.8) | 149 (35.9) | <0.001 |
| Arthralgia | 450 (9.3) | 148 (16.0) | 87 (21.0) | <0.001 |
| Anaphylactic shock | 11 (0.2) | 3 (0.3) | 5 (1.2) | 0.003 |
| None | 457 (9.5) | 76 (8.2) | 30 (7.2) | 0.186 |
| Crude OR | 95% CI | p-Value | Adjusted * OR | 95% CI | p-Value | Eta ** | |
|---|---|---|---|---|---|---|---|
| Sex | 0.81 | 0.71–0.91 | 0.001 | 0.95 | 0.81–1.10 | 0.483 | 0.07 |
| Age | 1.46 | 1.40–1.52 | <0.001 | 1.39 | 1.31–1.47 | <0.001 | |
| Child | 1.73 | 1.53–1.95 | <0.001 | 0.98 | 0.83–1.15 | 0.777 | |
| Annual household income | 1.05 | 0.91–1.22 | 0.526 | 1.27 | 1.08–1.49 | 0.003 | |
| Underlying disease | 1.94 | 1.69–2.22 | <0.001 | 1.30 | 1.10–1.54 | 0.003 | |
| Social norms | 3.98 | 3.66–4.34 | <0.001 | 4.02 | 3.64–4.45 | <0.001 | 0.27 |
| Confidence | 1.80 | 1.71–1.90 | <0.001 | 1.76 | 1.65–1.87 | <0.001 | 0.13 |
| Complacency | 2.34 | 2.20–2.49 | <0.001 | 2.18 | 2.03–2.34 | <0.001 | 0.18 |
| Constraints | 2.41 | 2.26–2.56 | <0.001 | 2.27 | 2.11–2.45 | <0.001 | 0.18 |
| Calculation | 1.12 | 1.07–1.17 | <0.001 | 1.10 | 1.04–1.16 | 0.001 | 0.07 |
| Collective responsibility | 1.83 | 1.73–1.93 | <0.001 | 1.77 | 1.65–1.90 | <0.001 | 0.13 |
| Compliance | 1.25 | 1.19–1.31 | <0.001 | 1.24 | 1.18–1.31 | <0.001 | 0.08 |
| Conspiracy | 1.53 | 1.45–1.62 | <0.001 | 1.42 | 1.33–1.52 | <0.001 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokiya, M.; Hara, M.; Matsumoto, A.; Ashenagar, M.S.; Nakano, T.; Hirota, Y. Acceptance of Booster COVID-19 Vaccine and Its Association with Components of Vaccination Readiness in the General Population: A Cross-Sectional Survey for Starting Booster Dose in Japan. Vaccines 2022, 10, 1102. https://doi.org/10.3390/vaccines10071102
Tokiya M, Hara M, Matsumoto A, Ashenagar MS, Nakano T, Hirota Y. Acceptance of Booster COVID-19 Vaccine and Its Association with Components of Vaccination Readiness in the General Population: A Cross-Sectional Survey for Starting Booster Dose in Japan. Vaccines. 2022; 10(7):1102. https://doi.org/10.3390/vaccines10071102
Chicago/Turabian StyleTokiya, Mikiko, Megumi Hara, Akiko Matsumoto, Mohammad Said Ashenagar, Takashi Nakano, and Yoshio Hirota. 2022. "Acceptance of Booster COVID-19 Vaccine and Its Association with Components of Vaccination Readiness in the General Population: A Cross-Sectional Survey for Starting Booster Dose in Japan" Vaccines 10, no. 7: 1102. https://doi.org/10.3390/vaccines10071102
APA StyleTokiya, M., Hara, M., Matsumoto, A., Ashenagar, M. S., Nakano, T., & Hirota, Y. (2022). Acceptance of Booster COVID-19 Vaccine and Its Association with Components of Vaccination Readiness in the General Population: A Cross-Sectional Survey for Starting Booster Dose in Japan. Vaccines, 10(7), 1102. https://doi.org/10.3390/vaccines10071102







