COVID-19 Vaccine Effectiveness against Omicron Variant among Underage Subjects: The Veneto Region’s Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
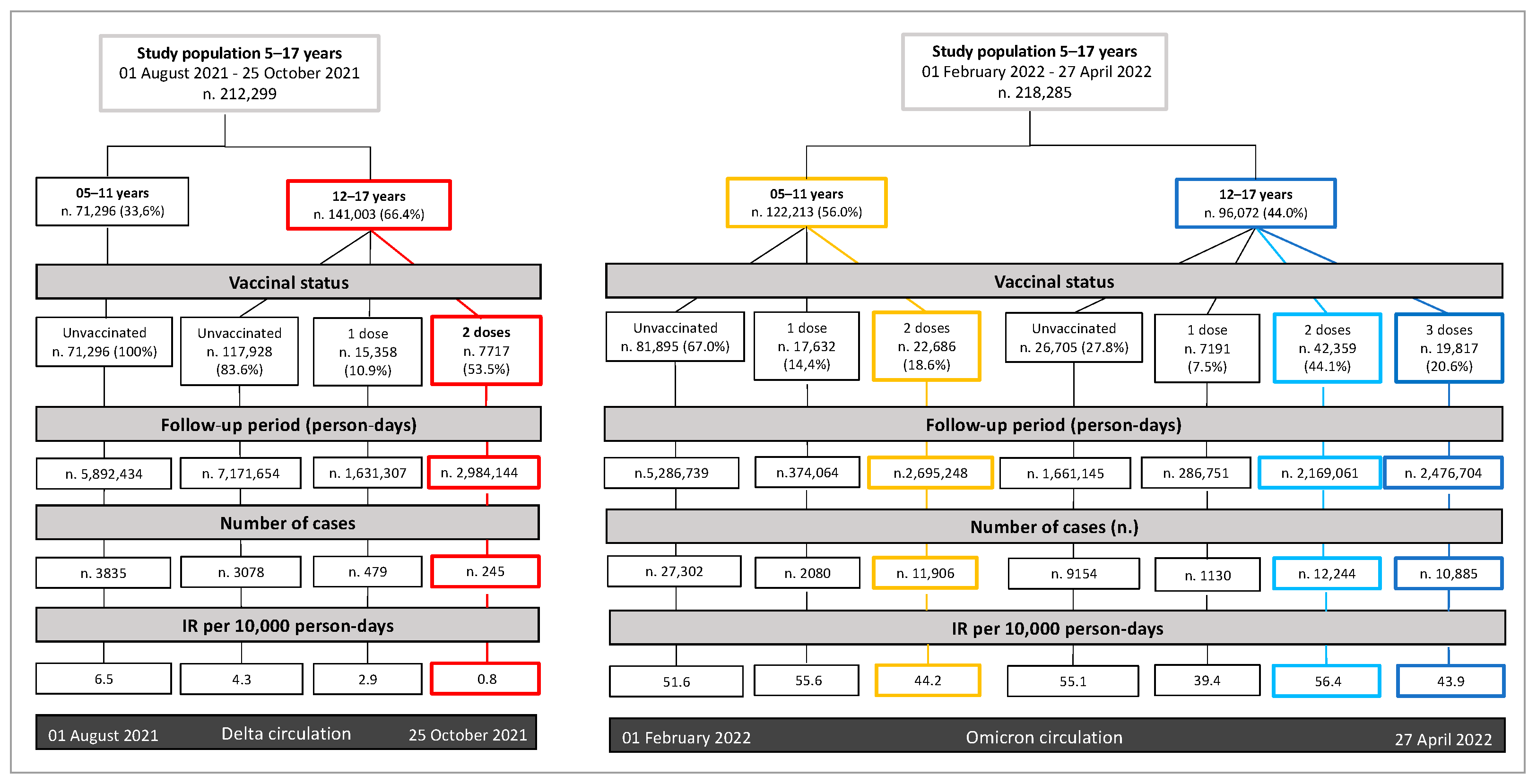
3.1. Vaccine Coverage and Characteristics of Study Populations at the Baseline
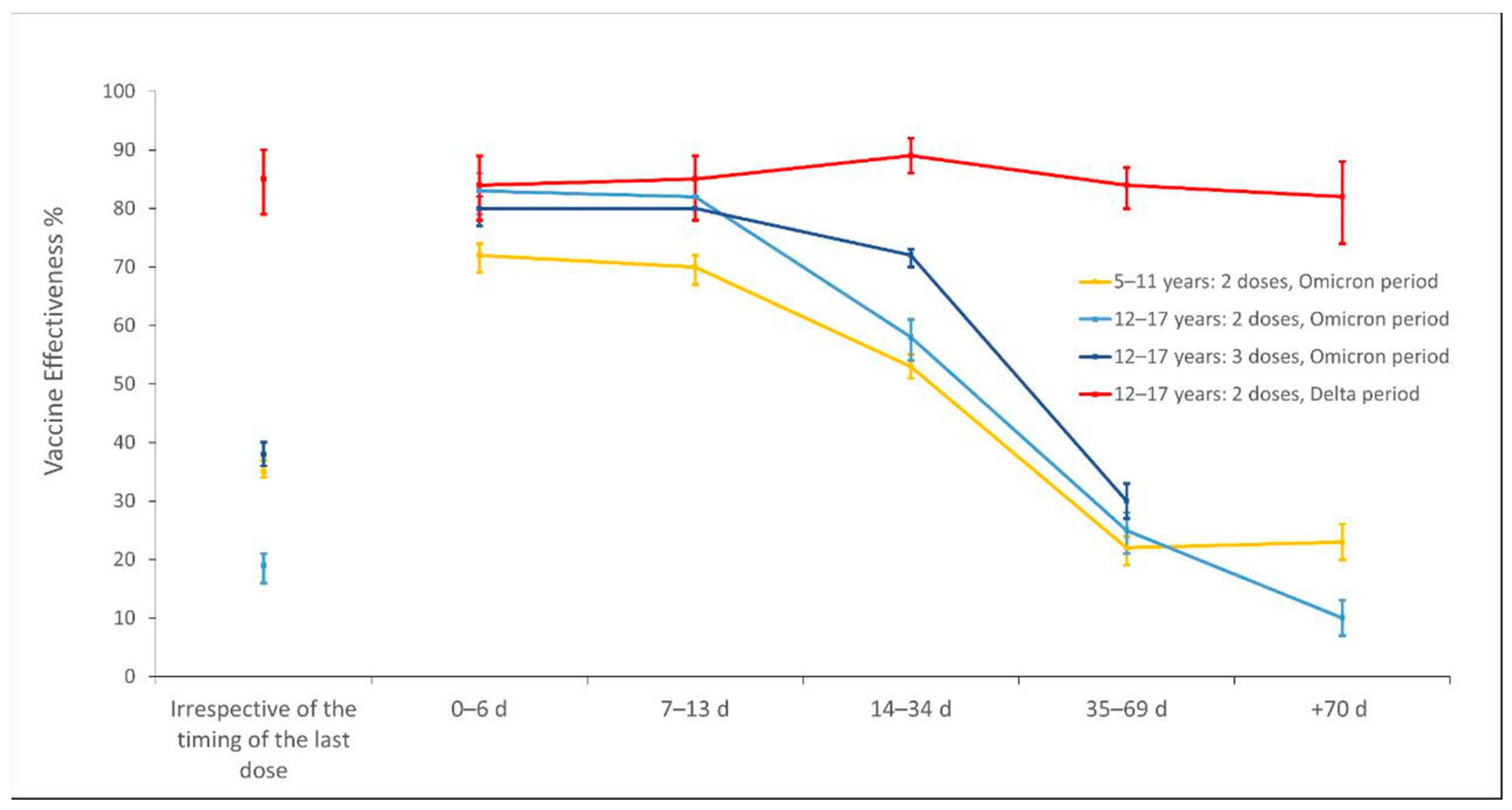
3.2. Incidence of SARS-CoV-2 Infections per Vaccine Status and Vaccine Effectiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Italian Department of Civil Protection. COVID-19 Italy—Monitoring of the Situation. Available online: https://opendatadpc.maps.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1 (accessed on 14 July 2022).
- Italian National Institute of Health (ISS). CovidStat INFN-Data from ISS. Available online: https://covid19.infn.it/iss/ (accessed on 14 July 2022).
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Interim Statement on COVID-19 Vaccination for Children and Adolescents. Available online: https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-vaccination-for-children-and-adolescents (accessed on 21 July 2022).
- Italian National Institute of Health (ISS). Extended Report. COVID-19: Surveillance, Impact, and Efficacy of Vaccinations. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_15-giugno-2022.pdf?fs=e&s=cl (accessed on 14 July 2022).
- Howard-Jones, A.R.; Burgner, D.P.; Crawford, N.W.; Goeman, E.; E Gray, P.; Hsu, P.; Kuek, S.; McMullan, B.J.; Tosif, S.; Wurzel, D.; et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J. Paediatr. Child Health 2022, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Govil-Dalela, T.; Sivaswamy, L. Neurological Effects of COVID-19 in Children. Pediatr. Clin. N. Am. 2021, 68, 1081–1091. [Google Scholar] [CrossRef]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Baggio, S.; L’Huillier, A.G.; Yerly, S.; Bellon, M.; Wagner, N.; Rohr, M.; Huttner, A.; Blanchard-Rohner, G.; Loevy, N.; Kaiser, L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load in the Upper Respiratory Tract of Children and Adults With Early Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, 148–150. [Google Scholar] [CrossRef]
- Buja, A.; Zabeo, F.; Cristofori, V.; Paganini, M.; Baldovin, T.; Fusinato, R.; Boccuzzo, G.; Cocchio, S.; Coretti, S.; Rebba, V.; et al. Opening Schools and Trends in SARS-CoV-2 Transmission in European Countries. Int. J. Public Health 2021, 66, 1604076. [Google Scholar] [CrossRef]
- Bhatt, M.; Plint, A.C.; Tang, K.; Malley, R.; Huy, A.P.; McGahern, C.; Dawson, J.; Pelchat, M.; Dawson, L.; Varshney, T.; et al. Household transmission of SARS-CoV-2 from unvaccinated asymptomatic and symptomatic household members with confirmed SARS-CoV-2 infection: An antibody-surveillance study. CMAJ Open 2022, 10, E357–E366. [Google Scholar] [CrossRef]
- Tönshoff, B.; Müller, B.; Elling, R.; Renk, H.; Meissner, P.; Hengel, H.; Garbade, S.F.; Kieser, M.; Jeltsch, K.; Grulich-Henn, J.; et al. Prevalence of SARS-CoV-2 Infection in Children and Their Parents in Southwest Germany. JAMA Pediatr. 2021, 175, 586–593. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Magaziotou, I.; Dedoukou, X.; Eleftheriou, E.; Raftopoulos, V.; Michos, A.; Lourida, A.; Panopoulou, M.; Stamoulis, K.; Papaevangelou, V.; et al. Children and Adolescents with SARS-CoV-2 Infection: Epidemiology, Clinical Course and Viral Loads. Pediatric Infect. Dis. J. 2020, 39, E388–E392. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Hwang, M.-J.; Kim, Y.-Y.; Park, S.Y.; Yoo, M.; Kim, S.-S.; Lee, S.; Kwon, D. Epidemiological Characteristics and Transmission Patterns of COVID-19 Cases Among Children and Adolescents Aged 0–18 Years in South Korea. Risk Manag. Healthc. Policy 2022, 15, 219–227. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA. 21 April 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-deployment-plans (accessed on 8 August 2022).
- Haute Autorité de Santé (HAS). Stratégie de Vaccination Contre la Covid-19—Place du Vaccin à ARNm COMIRNATY® Chez les 5–11 ans. 20 December 2021. Available online: https://webzine.has-sante.fr/jcms/p_3269889/en/strategie-de-vaccination-contre-la-covid-19-place-du-vaccin-a-arnm-comirnaty-chez-les-12-15-ans (accessed on 8 August 2022).
- Council of Ministers. Special Commissioners for the Implementation and Coordination of Public Health Policies to Contain and Contrast the Emergency Situations due to COVID-19 Epidemic. Anti-COVID Vaccination Campaign (13 March 2021). Available online: https://www.governo.it/it/approfondimento/piano-vaccinale-anti-covid-19/16510 (accessed on 14 July 2022).
- Italian Drug Agency (AIFA). Extension of Therapeutic Directions for Medicines; Centralized Procedures; Rep. 73/2021; AIFA: Rome, Italy, 2021.
- Italian Drug Agency (AIFA). Extension of Therapeutic Directions for Medicines; Centralized Procedures; Rep.111/2021; AIFA: Rome, Italy, 2021.
- Communication from the Ministry of Health. Object: Use Indications Extension of Comirnaty (BioNTech/Pfizer) Vaccine to the 5–11 Age-Group. Available online: https://www.aifa.gov.it/trova-farmaco (accessed on 13 July 2022).
- Communication from the Ministry of Health. Object: Further Extension of the Subpopulation Eligible to Receive Third Additional (“Booster”) Dose in the Context of the Anti-SARS-CoV-2/COVID-19 Vaccination Campaign. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=84693&parte=1%20&serie=null (accessed on 14 July 2022).
- Communication from the Ministry of Health. Object: Extension of the Recommendation of Third Additional (“Booster”) Dose to All the 12–15 Age Group in the Context of the Anti-SARS-CoV-2/COVID-19 Vaccination Campaign. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=84873&parte=1%20&serie=null (accessed on 14 July 2022).
- National Institute of Statistics (ISTAT). Age Groups by Sex: Veneto Region. Old Age Index and Mean Inhabitants Age. Available online: https://ugeo.urbistat.com/AdminStat/it/it/demografia/eta/veneto/5/2 (accessed on 14 July 2022).
- Christner, N.; Essler, S.; Hazzam, A.; Paulus, M. Children’s psychological well-being and problem behavior during the COVID-19 pandemic: An online study during the lockdown period in Germany. PLoS ONE 2021, 16, e0253473. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Helland, M.S.; Holt, T. The impact of school closure and social isolation on children in vulnerable families during COVID-19: A focus on children’s reactions. Eur. Child Adolesc. Psychiatry 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Meo, S.A.; Meo, A.S.; Al-Jassir, F.F.; Klonoff, D.C. Omicron SARS-CoV-2 New Variant: Global Prevalence and Biological and Clinical Characteristics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8012–8018. [Google Scholar]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Cocchio, S.; Zabeo, F.; Facchin, G.; Piva, N.; Venturato, G.; Marcon, T.; Saia, M.; Tonon, M.; Mongillo, M.; Da Re, F.; et al. Differences in Immunological Evasion of the Delta (B.1.617.2) and Omicron (B.1.1.529) SARS-CoV-2 Variants: A Retrospective Study on the Veneto Region’s Population. Int. J. Environ. Res. Public Health 2022, 19, 8179. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef]
- Wang, W.-C.; Fann, J.C.-Y.; Chang, R.-E.; Jeng, Y.-C.; Hsu, C.-Y.; Chen, H.-H.; Liu, J.-T.; Yen, A.M.-F. Economic evaluation for mass vaccination against COVID-19. J. Formos. Med. Assoc. 2021, 120, S95–S105. [Google Scholar] [CrossRef]
- Italian National Institute of Health (ISS). Prevalence and Distribution of SARS-CoV-2 Variants of Interest for the Public Health in Italy. Communication N. 08/2022—Flash Survey. Available online: https://www.iss.it/primo-piano/-/asset_publisher/3f4alMwzN1Z7/content/id/6608164 (accessed on 14 July 2022).
- Detail on the Deliberation of the Regional Council—Veneto Region Official Bulletin. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDgr.aspx?id=431849 (accessed on 25 July 2022).
- 9th Update on the Genetic Characteristics of SARS-CoV-2 Cases Identified in the Veneto Region. Available online: https://www.izsvenezie.it/caratteristiche-genetiche-sars-cov-2-veneto-9/ (accessed on 25 July 2022).
- 10th Update on the Genetic Characteristics of SARS-CoV-2 Cases Identified in the Veneto Region. Available online: https://www.izsvenezie.it/caratteristiche-genetiche-sars-cov-2-veneto-10/ (accessed on 25 July 2022).
- 12th Update on the Genetic Characteristics of SARS-CoV-2 Cases Identified in the Veneto Region. Available online: https://www.izsvenezie.it/caratteristiche-genetiche-sars-cov-2-veneto-12/ (accessed on 25 July 2022).
- 13th Update on the Genetic Characteristics of SARS-CoV-2 Cases Identified in the Veneto Region. Available online: https://www.izsvenezie.it/caratteristiche-genetiche-sars-cov-2-veneto-13/ (accessed on 25 July 2022).
- Veneto Region Council. Temporary Indications for the Priority Use of SARS-CoV-2 Molecular Tests, 29 December 2021. Available online: https://citynews-veronasera.stgy.ovh/~{}media/7262534639411/nota-tamponi-1-2.pdf (accessed on 20 March 2022).
- Lifelines Library (Python). Time Varying Survival Regression—Lifelines 0.27.1 Documentation. Available online: https://lifelines.readthedocs.io/en/latest/Time%20varying%20survival%20regression.html (accessed on 26 July 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Veneto Region Website. Vaccinations on the Veneto Region. Available online: https://www.regione.veneto.it/dati-vaccinazioni/ (accessed on 14 July 2022).
- Montalti, M.; Rallo, F.; Guaraldi, F.; Bartoli, L.; Po, G.; Stillo, M.; Perrone, P.; Squillace, L.; Dallolio, L.; Pandolfi, P.; et al. Would parents get their children vaccinated against SARS-CoV-2? Rate and predictors of vaccine hesitancy according to a survey over 5000 families from Bologna, Italy. Vaccines 2021, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Almalki, O.S.; Alfayez, O.M.; Al Yami, M.S.; Asiri, Y.A.; Almohammed, O.A. Parents’ Hesitancy to Vaccinate Their 5–11-Year-Old Children Against COVID-19 in Saudi Arabia: Predictors From the Health Belief Model. Front. Public Health 2022, 10, 842862. [Google Scholar] [CrossRef]
- Del Giudice, G.M.; Napoli, A.; Corea, F.; Folcarelli, L.; Angelillo, I.F. Evaluating COVID-19 Vaccine Willingness and Hesitancy among Parents of Children Aged 5–11 Years with Chronic Conditions in Italy. Vaccines 2022, 10, 396. [Google Scholar] [CrossRef]
- Imamura, T.; Saito, M.; Ko, Y.K.; Imamura, T.; Otani, K.; Akaba, H.; Ninomiya, K.; Furuse, Y.; Miyahara, R.; Sando, E.; et al. Roles of Children and Adolescents in COVID-19 Transmission in the Community: A Retrospective Analysis of Nationwide Data in Japan. Front. Pediatrics 2021, 9, 705882. [Google Scholar] [CrossRef] [PubMed]
- Cocchio, S.; Zabeo, F.; Facchin, G.; Piva, N.; Furlan, P.; Nicoletti, M.; Saia, M.; Tonon, M.; Mongillo, M.; Russo, F.; et al. The Effectiveness of a Diverse COVID-19 Vaccine Portfolio and Its Impact on the Persistence of Positivity and Length of Hospital Stays: The Veneto Region’s Experience. Vaccines 2022, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Del Manso, M.; Mateo-Urdiales, A.; Rota, M.C.; Petrone, D.; Riccardo, F.; Bella, A.; Siddu, A.; Battilomo, S.; Proietti, V.; et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: A retrospective analysis of January–April, 2022. Lancet 2022, 400, 97–103. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; Le Clair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5-11 Years and Adolescents Aged 12-15 Years - PROTECT Cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA J. Am. Med. Assoc. 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Molteni, E.; Canas, L.S.; Kläser, K.; Deng, J.; Bhopal, S.S.; Hughes, R.C.; Chen, L.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. Post-vaccination infection rates and modification of COVID-19 symptoms in vaccinated UK school-aged children and adolescents: A prospective longitudinal cohort study. Lancet Reg. Heal. Eur. 2022, 19, 100429. [Google Scholar] [CrossRef]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wölfl-Duchek, M.; Bergmann, F.; Jorda, A.; Weber, M.; Müller, M.; Seitz, T.; Zoufaly, A.; Strassl, R.; Zeitlinger, M.; Herkner, H.; et al. Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: A Diagnostic Accuracy Study. Microbiol Spectr. 2022, 10, e02029-21. [Google Scholar] [CrossRef]
- Cattelan, A.M.; Sasset, L.; Zabeo, F.; Ferrari, A.; Rossi, L.; Mazzitelli, M.; Cocchio, S.; Baldo, V. Rapid Antigen Test LumiraDxTM vs. Real Time Polymerase Chain Reaction for the Diagnosis of SARS-CoV-2 Infection: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 3826. [Google Scholar] [CrossRef] [PubMed]
| Characteristic of the Baseline Study Population | Delta Period 1 August 2021–25 October 2021 | Omicron Period 1 February 2022–27 April 2022 | ||||
|---|---|---|---|---|---|---|
| Overall (N = 212,299) | Negatives (N = 204,662) | Positives (N = 7637) | Overall (N = 218,285) | Negatives (N = 143,586) | Positives (N = 74,699) | |
| Percentage over the whole population | 100% | 96.4% | 3.6% | 100% | 65.8% | 34.2% |
| Sex * | ||||||
| Females ** | 98,277 | 94,734 (96.4%) | 3543 (3.6%) | 106,038 | 68,623 (64.7%) | 37,415 (35.3%) |
| Males | 114,022 | 109,928 (96.4%) | 4094 (3.6%) | 112,247 | 74,963 (66.8%) | 37,284 (33.2%) |
| Age class | ||||||
| 05–11 | 71,296 | 67,461 (94.6%) | 3835 (5.4%) | 122,213 | 80,927 (66.2%) | 41,286 (33.8%) |
| 12–17 | 141,003 | 137,201 (97.3%) | 3802 (2.7%) | 96,072 | 62,659 (65.2%) | 33,413 (34.8%) |
| Prior infection | ||||||
| No | 199,435 | 191,391 (96.2%) | 7504 (3.8%) | 198,871 | 128,177 (64.5%) | 70,694 (35.5%) |
| Yes | 12,864 | 12,731 (99%) | 133 (1%) | 19,414 | 15,409 (79.4%) | 4005 (20.6%) |
| Vaccine status | ||||||
| Unvaccinated | 189,223 | 181,876 (96.1%) | 7347 (3.9%) | 108,6 | 71,124 (65.5%) | 37,476 (34.5%) |
| First dose | ||||||
| BNT162b2 *** | 15,181 | 14,953 (98.5%) | 228 (1.5%) | 22,777 | 16,439 (72.2%) | 6338 (27.8%) |
| mRNA-1273 | 178 | 175 (98.3%) | 3 (1.7%) | 2046 | 1490 (72.8%) | 556 (27.2%) |
| Second dose | ||||||
| BNT162b2 | 7671 | 7612 (99.2%) | 59 (0.8%) | 56,83 | 37,086 (65.3%) | 19,744 (34.7%) |
| mRNA-1273 | 46 | 46 (100%) | 0 (0%) | 8215 | 5408 (65.8%) | 2807 (34.2%) |
| Third dose | ||||||
| BNT162b2 | 18,353 | 11,170 (60.9%) | 7183 (39.1%) | |||
| Primary cycle with mRNA-1273 + BNT162b2 | 1464 | 869 (59.4%) | 595 (40.6%) | |||
| Vaccine Status and Number of Days from the Last Dose Received, Stratified by Age Class | Delta Period | Omicron Period | ||
|---|---|---|---|---|
| Incidence (×10,000 Person-Days) | Vaccine Effectiveness % (95% CI) | Incidence (×10,000 Person-Days) | Vaccine Effectiveness % (95% CI) | |
| Age class 05–11 * | ||||
| Unvaccinated ** | 51.6 | Ref. | ||
| Second dose | ||||
| BNT162b2 *** | ||||
| Irrespective of the timing of the last dose **** | 44.2 | 35 (34–37) | ||
| 0–6 d | 33.4 | 72 (69–74) | ||
| 7–13 d | 35.6 | 70 (67–72) | ||
| 14–34 d | 36.6 | 53 (51–55) | ||
| 35–69 d | 46.8 | 22 (19–24) | ||
| +70 d | 56.9 | 23 (20–26) | ||
| Age class 12–17 | ||||
| Unvaccinated | 4.3 | Ref. | 55.1 | Ref. |
| Second dose | ||||
| Any vaccine irrespective of the timing of the last dose | 0.8 | 86 (84–88) | 56.4 | 19 (16–21) |
| BNT162b2 | ||||
| Irrespective of the timing of the last dose | 0.9 | 85 (83–87) | 57.5 | 17 (15–20) |
| 0–6 d | 1.0 | 83 (76–88) | 24.5 | 81 (76–85) |
| 7–13 d | 1.0 | 84 (77–89) | 21.7 | 83 (79–86) |
| 14–34 d | 0.7 | 88 (85–91) | 34.9 | 59 (55–62) |
| 35–69 d | 1.0 | 83 (79–87) | 51.2 | 23 (19–27) |
| +70 d | 1.0 | 82 (74–88) | 65.1 | 8 (5–11) |
| mRNA-1273 | ||||
| Irrespective of the timing of the last dose | 0.4 | 94 (88–97) | 52.8 | 30 (26–33) |
| 0–6 d | 0.6 | 90 (69–97) | 15.9 | 88 (81–92) |
| 7–13 d | 0.6 | 90 (68–97) | 29.4 | 78 (69–84) |
| 14–34 d | 0.3 | 96 (86–99) | 40.3 | 55 (49–61) |
| 35–69 d | 0 | ns | 49.6 | 29 (23–35) |
| +70 d | 0 | ns | 58.5 | 20 (15–24) |
| Third dose | ||||
| Any vaccine irrespective of the timing of the last dose | 43.9 | 38 (36–40) | ||
| BNT162b2 | ||||
| Irrespective of the timing of the last dose | 44.3 | 38 (36–40) | ||
| 0–6 d | 27.8 | 79 (77–81) | ||
| 7–13 d | 26.0 | 80 (78–82) | ||
| 14–34 d | 24.7 | 72 (70–73) | ||
| 35–69 d | 48.4 | 30 (27–33) | ||
| +70 d | 77.0 | ns | ||
| Primary cycle with mRNA-1273 + BNT162b2 | ||||
| Irrespective of the timing of the last dose | 40.4 | 53 (50–56) | ||
| 0–6 d | 23.9 | 82 (77–86) | ||
| 7–13 d | 26.4 | 80 (75–84) | ||
| 14–34 d | 25.5 | 71 (66–74) | ||
| 35–69 d | 48.1 | 32 (26–38) | ||
| +70 d | 79.9 | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchio, S.; Zabeo, F.; Tremolada, G.; Facchin, G.; Venturato, G.; Marcon, T.; Saia, M.; Tonon, M.; Mongillo, M.; Da Re, F.; et al. COVID-19 Vaccine Effectiveness against Omicron Variant among Underage Subjects: The Veneto Region’s Experience. Vaccines 2022, 10, 1362. https://doi.org/10.3390/vaccines10081362
Cocchio S, Zabeo F, Tremolada G, Facchin G, Venturato G, Marcon T, Saia M, Tonon M, Mongillo M, Da Re F, et al. COVID-19 Vaccine Effectiveness against Omicron Variant among Underage Subjects: The Veneto Region’s Experience. Vaccines. 2022; 10(8):1362. https://doi.org/10.3390/vaccines10081362
Chicago/Turabian StyleCocchio, Silvia, Federico Zabeo, Giulia Tremolada, Giacomo Facchin, Giovanni Venturato, Thomas Marcon, Mario Saia, Michele Tonon, Michele Mongillo, Filippo Da Re, and et al. 2022. "COVID-19 Vaccine Effectiveness against Omicron Variant among Underage Subjects: The Veneto Region’s Experience" Vaccines 10, no. 8: 1362. https://doi.org/10.3390/vaccines10081362
APA StyleCocchio, S., Zabeo, F., Tremolada, G., Facchin, G., Venturato, G., Marcon, T., Saia, M., Tonon, M., Mongillo, M., Da Re, F., Russo, F., & Baldo, V. (2022). COVID-19 Vaccine Effectiveness against Omicron Variant among Underage Subjects: The Veneto Region’s Experience. Vaccines, 10(8), 1362. https://doi.org/10.3390/vaccines10081362







