Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of mRNA COVID-19 Vaccine: The MOSAICO Study
Abstract
:1. Introduction
2. Materials and Methods
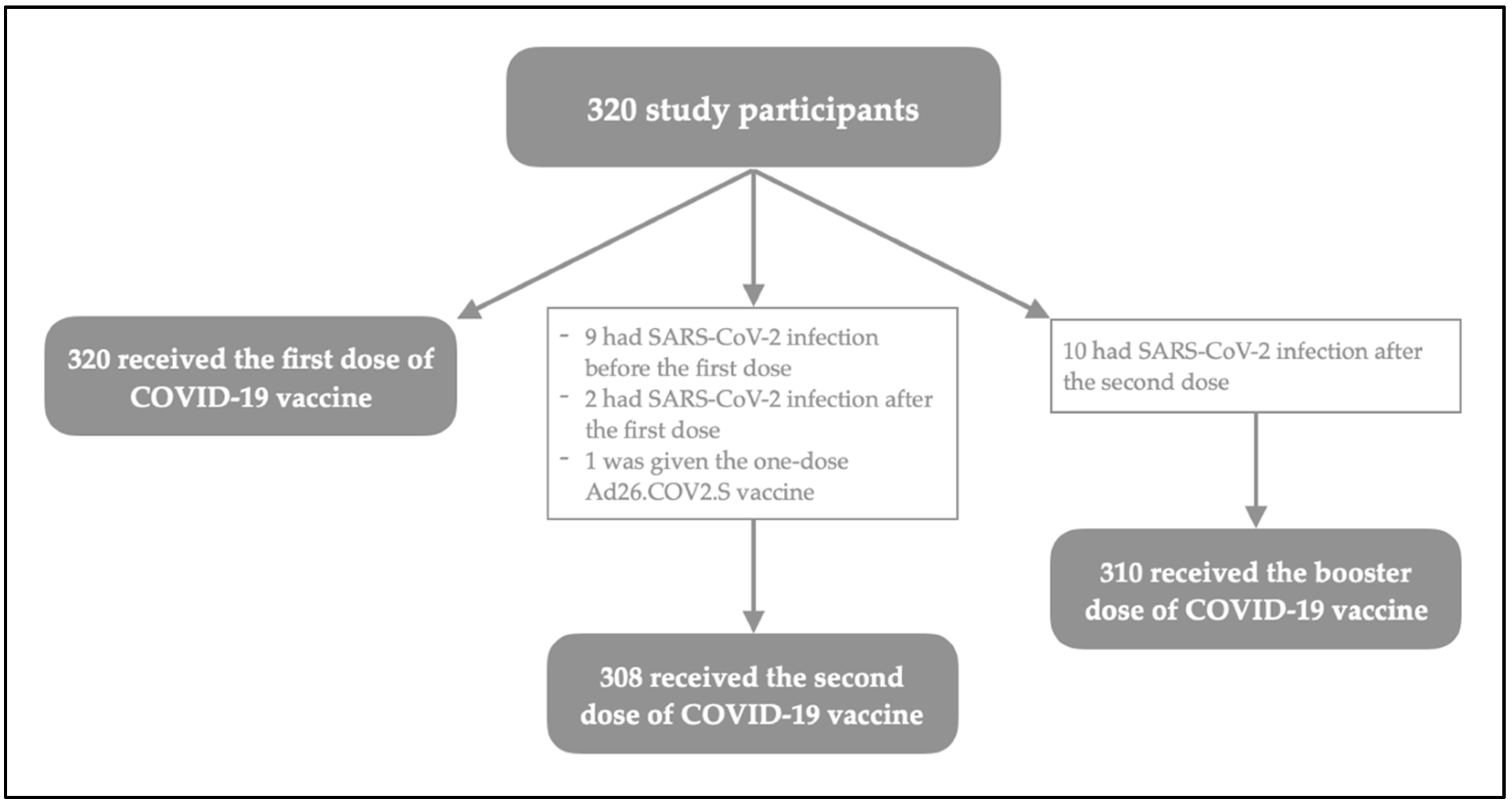
2.1. Study Design and Population
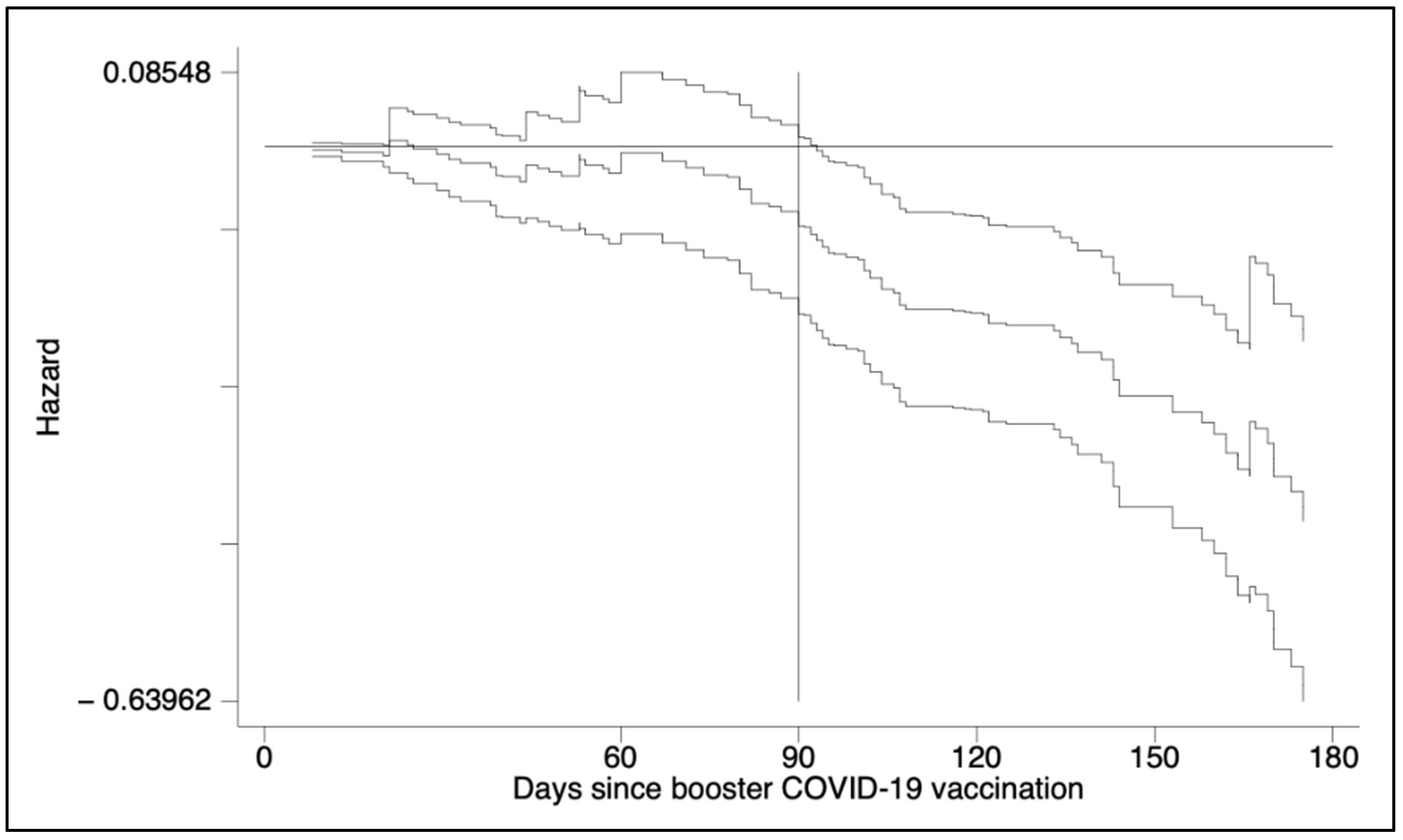
2.2. Statistical Analysis
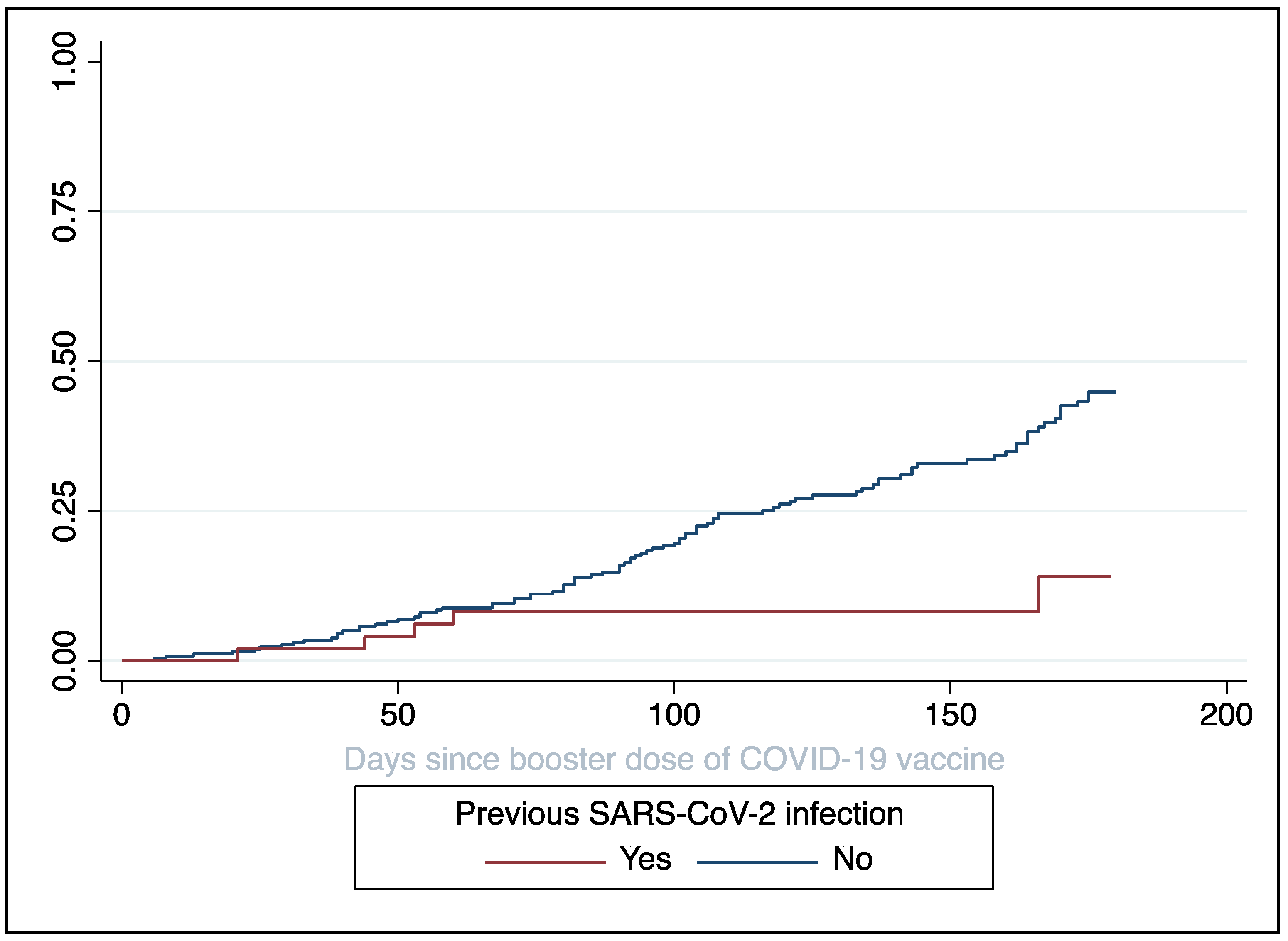
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Antonazzo, I.C.; Polosa, R. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up—Reply. Intern. Emerg. Med. 2022, 17, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel. Med. 2021, 28, taab173. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute. Piano Vaccini Anti COVID-19. Published Online. 2021. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5452&area=nuovoCoronavirus&menu=vuoto (accessed on 29 June 2022).
- Dopfer-Jablonka, A.; Steffens, S.; Müller, F.; Mikuteit, M.; Niewolik, J.; Cossmann, A.; Stankov, M.V.; Behrens, G.M.N.; Hummers, E.; Heesen, G.; et al. SARS-CoV-2-specific immune responses in elderly and immunosuppressed participants and patients with hematologic disease or checkpoint inhibition in solid tumors: Study protocol of the prospective, observational CoCo immune study. BMC Infect. Dis. 2022, 22, 403. [Google Scholar] [CrossRef]
- Ponticelli, D.; Madotto, F.; Conti, S.; Antonazzo, I.C.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; et al. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up. Intern. Emerg. Med. 2021, 17, 481–486. [Google Scholar] [CrossRef]
- Della Valle, P.; Fabbri, M.; Madotto, F.; Ferrara, P.; Cozzolino, P.; Calabretto, E.; D’Orso, M.; Longhi, E.; Polosa, R.; Riva, M.; et al. Occupational Exposure in the Lombardy Region (Italy) to SARS-CoV-2 Infection: Results from the MUSTANG–OCCUPATION–COVID-19 Study. Int. J. Environ. Res. Public. Health 2021, 18, 2567. [Google Scholar] [CrossRef]
- Ferrara, P.; Albano, L. COVID-19 and healthcare systems: What should we do next? Public Health 2020, 185, 1–2. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Veneri, C.; Mancini, P.; Bonanno Ferraro, G.; Brandtner, D.; Lucentini, L.; Bonadonna, L.; Rossi, M.; Grigioni, M.; et al. The rapid spread of SARS-CoV-2 Omicron variant in Italy reflected early through wastewater surveillance. Sci. Total Environ. 2022, 837, 155767. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, ciac262. [Google Scholar] [CrossRef] [PubMed]
- Biggs, A.T.; Littlejohn, L.F. Vaccination and natural immunity: Advantages and risks as a matter of public health policy. Lancet Reg. Health Am. 2022, 8, 100242. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Royston, P. Using Aalen’s Linear Hazards Model to Investigate Time-varying Effects in the Proportional Hazards Regression Model. Stata J. Promot. Commun. Stat. Stata 2002, 2, 331–350. [Google Scholar] [CrossRef]
- Presidenza del Consiglio dei Ministri-Dipartimento di Protezione Civile. Risorse Dati Su COVID-19. Available online: https://dati-covid.italia.it (accessed on 29 June 2022).
- d’Alessandro, V.; Balasco, N.; Ferrara, P.; Vitagliano, L. The temporal correlation between positive testing and death in Italy: From the first phase to the later evolution of the COVID-19 pandemic: Time evolution of COVID-19 weekly lethality rate. Acta Biomed. Atenei Parm. 2022, 92, e2021395. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- R Core Team. R 4.2.0; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Oster, Y.; Benenson, S.; Nir-Paz, R.; Buda, I.; Cohen, M.J. The effect of a third BNT162b2 vaccine on breakthrough infections in health care workers: A cohort analysis. Clin. Microbiol. Infect. 2022, 28, 735.e1–735.e3. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Monitoraggio Fase 2 Report Settimanale (Aggiornati al 30/03/2022); Istituto Superiore di Sanità: Rome, Italy, 2022. Available online: https://www.iss.it/documents/20126/0/Monitoraggio+Fase+2_+report_nazionale_98_finale.pdf/78df4d85-1ef1-34c1-bb52-597d01397358?t=1648829173984 (accessed on 19 July 2022).
- Cerqueira-Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; de Araújo Oliveira, V.; Paixão, E.S.; Júnior, J.B.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: A test-negative, case-control study. Lancet Infect. Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M. COVID vaccine plus infection can lead to months of immunity. Nature 2022, d41586-022-00961–00963. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute. COVID-19. Published online 2022. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/homeNuovoCoronavirus.jsp (accessed on 19 July 2022).
- Polosa, R.; Tomaselli, V.; Ferrara, P.; Romeo, A.C.; Rust, S.; Saitta, D.; Caraci, F.; Romano, C.; Thangaraju, M.; Zuccarello, P.; et al. Seroepidemiological Survey on the Impact of Smoking on SARS-CoV-2 Infection and COVID-19 Outcomes: Protocol for the Troina Study. JMIR Res. Protoc. 2021, 10, e32285. [Google Scholar] [CrossRef]
- Tomaselli, V.; Ferrara, P.; Cantone, G.G.; Romeo, A.C.; Rust, S.; Saitta, D.; Caraci, F.; Romano, C.; Thangaraju, M.; Zuccarello, P.; et al. The effect of laboratory-verified smoking on SARS-CoV-2 infection: Results from the Troina sero-epidemiological survey. Intern. Emerg. Med. 2022, 1–4. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Slama, N.; Alexeeff, S.E.; Sakoda, L.C.; Fogelberg, R.; Myers, L.C.; Campbell, C.I.; Adams, A.S.; Prochaska, J.J. Tobacco Smoking and Risk of SARS-CoV-2 Infection and Disease Severity Among Adults in an Integrated Healthcare System in California. Nicotine Tob. Res. 2022, ntac090. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.C.; Mantovani, L.G.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Ferrara, P.; Albano, L. Azithromycin Has Been Flying Off the Shelves: The Italian Lesson Learnt from Improper Use of Antibiotics against COVID-19. Medicina 2022, 58, 363. [Google Scholar] [CrossRef]
| N (%) | |
|---|---|
| Total vaccinees | 320 |
| Age * | 38 (32–50) |
| Sex | |
| Male | 131 (40.9) |
| Female | 189 (59.1) |
| Role | |
| HCWs | 248 (77.5) |
| Non-HCWs | 72 (22.5) |
| Cigarette smoker | |
| Never | 178 (55.6) |
| Current | 121 (37.8) |
| Former | 21 (6.6) |
| E-cigarette user | 50 (15.6) |
| Of which, dual users | 49 (15.3) |
| Health status | |
| Previous SARS-CoV-2 infection | 60 (18.8) |
| Autoimmune disease | 10 (3.1) |
| Immunosuppressive therapy | 5 (1.6) |
| Variable | Hazard Ratio | SE | 95% CI | p-Value |
|---|---|---|---|---|
| Log likelihood = −507.17; χ2 = 27.39 (4 df); p-value < 0.0001 | ||||
| Previous infection with SARS-CoV-2 | 0.01 | |||
| No | Ref. | - | - | |
| Yes | 0.32 | 0.15 | 0.13–0.80 | |
| Age (continuous, in years) | 0.97 | 0.01 | 0.96–0.99 | 0.01 |
| Professional role | 0.001 | |||
| Non-HCWs | Ref. | - | - | |
| HCWs | 0.49 | 0.11 | 0.32–0.76 | |
| Smoking habits | 0.08 | |||
| Never/former smoker | Ref. | - | - | |
| Cigarette smoker | 0.61 | 0.17 | 0.35–1.06 | |
| E-cigarette user/Dual user | Omitted for p-value > 0.4 | |||
| N (%) | |
|---|---|
| Contact with confirmed COVID-19 case | 57 (57.6) |
| Time length from testing positive to negative (in days) * | 11.0 ± 3.6 |
| COVID-19 symptoms | |
| At least one | 79 (80.6) |
| Nasal congestion or runny nose | 35 (35.7) |
| Muscle/joint or body pains | 34 (34.7) |
| Fever/chills | 33 (33.7) |
| Cough | 31 (31.6) |
| Sore throat | 25 (25.5) |
| Headache | 20 (20.4) |
| Shortness of breath or difficulty in breathing | 10 (10.2) |
| Asthenia/fatigue/weakness | 8 (8.2) |
| Ageusia (loss of sense of taste) | 6 (6.1) |
| Anosmia (loss of smell) | 6 (6.1) |
| Tachyarrhythmia | 2 (2.0) |
| Vertigo | 2 (2.0) |
| Neuralgia | 1 (1.0) |
| Use of medication § | |
| At least one | 51 (53.1) |
| Paracetamol | 22 (22.9) |
| Non-steroidal anti-inflammatory drug | 9 (9.4) |
| Prednisone | 14 (14.6) |
| Betamethasone | 2 (2.1) |
| Dexamethasone | 1 (1.0) |
| Unknown glucocorticosteroid | 4 (4.2) |
| Azithromycin | 15 (15.6) |
| Amoxicillin/clavulanic acid | 3 (3.1) |
| Clarithromycin | 1 (1.0) |
| Unknown antibiotic | 5 (5.2) |
| Bromhexine | 1 (1.0) |
| Oxymetazoline | 1 (1.0) |
| C and/or D vitamin supplement | 21 (21.9) |
| Lactoferrin | 2 (2.1) |
| Model 1: Time-length between testing positive and negative (N = 98) | ||||
| Variable | Coefficient | SE | 95% CI | p-value |
| F(3,94) = 6.15; p-value = 0.0007; R2 = 0.16; adjusted R2 = 0.14 | ||||
| Autoimmune disease | 0.007 | |||
| No | Ref. | - | - | |
| Yes | 4.85 | 1.75 | 1.37–8.32 | |
| Sex | 0.02 | |||
| Male | Ref. | - | - | |
| Female | −1.60 | 0.70 | −2.98–−0.22 | |
| Smoking habits | 0.13 | |||
| Never/former smoker | Ref. | - | - | |
| Cigarette smoker | Omitted for p-value > 0.4 | - | - | |
| E-cigarette user/Dual user | 1.38 | 0.17 | −0.41–3.17 | |
| Model 2: Likelihood of develop at least a COVID-19 symptom during breakthrough infection (N = 89) | ||||
| Variable | Odds Ratio | SE | 95% CI | p-value |
| Log likelihood = −42.51; χ2 = 7.27 (3 df); p-value = 0.06 | ||||
| Age (continuous, in years) | 1.05 | 0.3 | 1.00–1.11 | 0.05 |
| Time-length between testing positive and negative (continuous, in days) | 1.11 | 0.10 | 0.94–1.32 | 0.23 |
| Smoking habits | 0.29 | |||
| Never/former smoker | Ref. | - | - | |
| Cigarette smoker | Omitted for p-value > 0.4 | - | - | |
| E-cigarette user/Dual user | 0.46 | 0.33 | 0.11–1.88 | |
| Model 3: Likelihood of using medications due to COVID-19 symptoms (N = 91) | ||||
| Variable | Odds Ratio | SE | 95% CI | p-value |
| Log likelihood = −53.23; χ2 = 19.67 (4 df); p-value = 0.0006 | ||||
| Presence of at least one COVID-19 symptom | 0.001 | |||
| No | Ref. | - | - | |
| Yes | 13.25 | 10.78 | 2.69–65.30 | |
| Sex | 0.08 | |||
| Male | Ref. | - | - | |
| Female | 2.38 | 1.18 | 0.91–6.27 | |
| Smoking habits | 0.31 | |||
| Never/former smoker | Ref. | - | - | |
| Cigarette smoker | 1.92 | 1.24 | 0.54–6.81 | |
| E-cigarette user/Dual user | Omitted for p-value > 0.4 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, P.; Ponticelli, D.; Magliuolo, R.; Borrelli, M.; Schiavone, B.; Mantovani, L.G. Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of mRNA COVID-19 Vaccine: The MOSAICO Study. Vaccines 2022, 10, 1353. https://doi.org/10.3390/vaccines10081353
Ferrara P, Ponticelli D, Magliuolo R, Borrelli M, Schiavone B, Mantovani LG. Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of mRNA COVID-19 Vaccine: The MOSAICO Study. Vaccines. 2022; 10(8):1353. https://doi.org/10.3390/vaccines10081353
Chicago/Turabian StyleFerrara, Pietro, Domenico Ponticelli, Roberto Magliuolo, Mario Borrelli, Beniamino Schiavone, and Lorenzo Giovanni Mantovani. 2022. "Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of mRNA COVID-19 Vaccine: The MOSAICO Study" Vaccines 10, no. 8: 1353. https://doi.org/10.3390/vaccines10081353
APA StyleFerrara, P., Ponticelli, D., Magliuolo, R., Borrelli, M., Schiavone, B., & Mantovani, L. G. (2022). Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of mRNA COVID-19 Vaccine: The MOSAICO Study. Vaccines, 10(8), 1353. https://doi.org/10.3390/vaccines10081353







