Exploring the Willingness of the COVID-19 Vaccine Booster Shots in China Using the Health Belief Model: Web-Based Online Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Characteristics of Participants’ COVID-19 Vaccination
3.3. HBM Predictive Factors of COVID-19 Booster Vaccination
4. Discussion
5. Conclusions
6. Limitations and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L. COVID-19 will continue but the end of the pandemic is near. Lancet 2022, 399, 417–419. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 June 2022).
- World Health Organization. Seventy-Third World Health Assembly. Available online: https://www.who.int/about/governance/world-health-assembly/seventy-third-world-health-assembly (accessed on 7 June 2022).
- Fontanet, A.; Cauchemez, S. COVID-19 herd immunity: Where are we? Nat. Rev. Immunol. 2020, 20, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Central People’s Government of the People’s Republic of China. Press Conference on the Joint Prevention and Control Mechanism of the State Council. Available online: http://www.nhc.gov.cn/xcs/s3574/202206/6cab6c76c3e345cda75044ec7826c361.shtml (accessed on 5 June 2022).
- Cheng, Z.J.; Huang, H.; Zheng, P.; Xue, M.; Ma, J.; Zhan, Z.; Gan, H.; Zeng, Y.; Lin, R.; Li, S. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy 2022, 77, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Kherabi, Y.; Fiolet, T.; Rozencwajg, S.; Salaün, J.-P.; Peiffer-Smadja, N. COVID-19 vaccine boosters: What do we know so far? Anaesth. Crit. Care Pain Med. 2021, 40, 100959. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.; Cravioto, A.; Rees, H.; Higgins, J.P.; Boutron, I. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against Covid-19 by BNT162b2 booster across age groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Juno, J.A.; Wheatley, A.K. Boosting immunity to COVID-19 vaccines. Nat. Med. 2021, 27, 1874–1875. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bao, L.; Wang, L.; Cao, L.; Chi, H.; Hu, Y.; Li, Q.; Zhou, Y.; Jiang, Y. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature 2022, 603, 919–925. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, H.; Zhang, Q.; Zhang, Y.; Lin, K.; Fu, Z.; Song, J.; Zhao, Y.; Fan, M.; Wang, H. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2022, 32, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Jiang, H.; Liu, Z.; Guo, Y.; Hu, D. The uptake and vaccination willingness of COVID-19 vaccine among Chinese residents: Web-based online cross-sectional study. Vaccines 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.Y.; Wang, R.T.; Tao, L.Y.; Liu, M.; Liu, J. Acceptance of a Third Dose of COVID-19 Vaccine and Associated Factors in China Based on Health Belief Model: A National Cross-Sectional Study. Vaccines 2022, 10, 89. [Google Scholar] [CrossRef]
- Guvenc, G.; Akyuz, A.; Acikel, C.H. Health Belief Model Scale for Cervical Cancer and Pap Smear Test: Psychometric testing. J. Adv. Nurs. 2011, 67, 428–437. [Google Scholar] [CrossRef]
- Dodel, M.; Mesch, G. Cyber-victimization preventive behavior: A health belief model approach. Comput. Hum. Behav. 2017, 68, 359–367. [Google Scholar] [CrossRef]
- Hubble, M.W.; Zontek, T.L.; Richards, M.E. Predictors of Influenza Vaccination among Emergency Medical Services Personnel. Prehospital Emerg. Care 2011, 15, 175–183. [Google Scholar] [CrossRef]
- Shahrabani, S.; Benzion, U. How Experience Shapes Health Beliefs: The Case of Influenza Vaccination. Health Educ. Behav. 2012, 39, 612–619. [Google Scholar] [CrossRef]
- Ziemer, K.S.; Hoffman, M.A. Beliefs and attitudes regarding human papillomavirus vaccination among college-age women. J. Health Psychol. 2013, 18, 1360–1370. [Google Scholar] [CrossRef]
- Oh, K.M.; Alqahtani, N.; Chang, S.; Cox, C. Knowledge, beliefs, and practice regarding human papillomavirus (HPV) vaccination among American college students: Application of the health belief model. J. Am. Coll. Health 2021, 1–10. [Google Scholar] [CrossRef]
- Dyda, A.; King, C.; Dey, A.; Leask, J.; Dunn, A.G. A systematic review of studies that measure parental vaccine attitudes and beliefs in childhood vaccination. BMC Public Health 2020, 20, 1253. [Google Scholar] [CrossRef] [PubMed]
- Carrion, M.L. An Ounce of Prevention: Identifying Cues to (In)Action for Maternal Vaccine Refusal. Qual. Health Res. 2018, 28, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Reindl, D.; Catma, S. A pre-vaccine analysis using the Health Belief Model to explain parents’ willingness to vaccinate (WTV) their children in the United States: Implications for vaccination programs. Expert Rev. Pharm. Outcomes Res. 2022, 22, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Deas, J.; Bean, S.J.; Sokolovska, I.; Fautin, C. Childhood Vaccine Attitudes and Information Sources Among Oregon Parents and Guardians. Health Promot. Pract. 2019, 20, 529–538. [Google Scholar] [CrossRef]
- Berg, M.B.; Lin, L.D. Predictors of COVID-19 vaccine intentions in the United States: The role of psychosocial health constructs and demographic factors. Transl. Behav. Med. 2021, 11, 1782–1788. [Google Scholar] [CrossRef]
- Okuyan, B.; Bektay, M.Y.; Demirci, M.Y.; Ay, P.; Sancar, M. Factors associated with Turkish pharmacists’ intention to receive COVID-19 vaccine: An observational study. Int. J. Clin. Pharm. 2022, 44, 247–255. [Google Scholar] [CrossRef]
- Alrajeh, A.M.; Daghash, H.; Buanz, S.F.; Altharman, H.A.; Belal, S. COVID-19 Vaccine Hesitancy Among the Adult Population in Saudi Arabia. Cureus 2021, 13, e20197. [Google Scholar] [CrossRef]
- Zampetakis, L.A.; Melas, C. The health belief model predicts vaccination intentions against COVID-19: A survey experiment approach. Appl. Psychol.-Health Well Being 2021, 13, 469–484. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Zhou, X.D.; Wang, H.Q.; Dong, S.X.; Wang, M.M.; Akezhuoli, H.; Zhu, H. COVID-19 vaccination intention and influencing factors among different occupational risk groups: A cross-sectional study. Hum. Vaccines Immunother. 2021, 17, 3433–3440. [Google Scholar] [CrossRef]
- Luo, W.; Song, S.Y. Perceived Benefits and Barriers to Chinese COVID-19 Vaccine Uptake Among Young Adults in China. Front. Public Health 2022, 10, 825874. [Google Scholar] [CrossRef]
- Shah, S.; Gui, H.; Chua, P.E.Y.; Tan, J.Y.; Suen, L.K.P.; Chan, S.W.C.; Pang, J.X. Factors associated with COVID-19 vaccination intent in Singapore, Australia and Hong Kong. Vaccine 2022, 40, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Kocoglu-Tanyer, D.; Dengiz, K.S.; Sacikara, Z. Development and psychometric properties of the public attitude towards vaccination scale—Health belief model. J. Adv. Nurs. 2020, 76, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Limbu, Y.B.; Gautam, R.K.; Pham, L. The Health Belief Model Applied to COVID-19 Vaccine Hesitancy: A Systematic Review. Vaccines 2022, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Central People’s Government of the People’s Republic of China. Press Conference on the Joint Prevention and Control Mechanism of the State Council. Available online: http://www.gov.cn/xinwen/gwylflkjz184/index.htm (accessed on 29 March 2022).
- Lai, X.Z.; Zhu, H.; Wang, J.H.; Huang, Y.Z.; Jing, R.Z.; Lyu, Y.; Zhang, H.J.; Feng, H.Y.F.; Guo, J.; Fang, H. Public Perceptions and Acceptance of COVID-19 Booster Vaccination in China: A Cross-Sectional Study. Vaccines 2021, 9, 1461. [Google Scholar] [CrossRef]
- Wang, Z.X.; Fang, Y.; Yu, F.Y.; Chan, P.S.F.; Chen, S.Y.; Sun, F.H. Facilitators and Barriers to Take up a COVID-19 Vaccine Booster Dose among Community-Dwelling Older Adults in Hong Kong: A Population-Based Random Telephone Survey. Vaccines 2022, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.C.; Fang, Y.; Chan, P.S.F.; Cao, H.; Chen, H.B.; Hu, T.; Chen, Y.Q.; Zhou, X.F.; Wang, Z.X. Behavioral Intention to Get a Booster Dose of COVID-19 Vaccine among Chinese Factory Workers. Int. J. Environ. Res. Public Health 2022, 19, 5245. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.Y.; Wang, R.T.; Tao, L.Y.; Liu, M.; Liu, J. Association Between Risk Perception and Acceptance for a Booster Dose of COVID-19 Vaccine to Children Among Child Caregivers in China. Front. Public Health 2022, 10, 834572. [Google Scholar] [CrossRef]
- Ai, J.; Wang, X.; He, X.; Zhao, X.; Zhang, Y.; Jiang, Y.; Li, M.; Cui, Y.; Chen, Y.; Qiao, R. Antibody resistance of SARS-CoV-2 Omicron BA. 1, BA. 1.1, BA. 2 and BA. 3 sub-lineages. bioRxiv 2022, 30, 1077–1083. [Google Scholar] [CrossRef]
- Hall, V.; Hopkins, S. COV-BOOST: Evidence to support rapid booster deployment. Lancet 2021, 398, 2209–2211. [Google Scholar] [CrossRef]
- Hung, I.F.; Poland, G.A. Single-dose Oxford–AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet 2021, 397, 854–855. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Chen, S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet 2022, 399, 2011–2012. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Lin, C.; Yang, Z.; Chow, C.; Lu, Z.; You, C. An Analysis of the Willingness to the COVID-19 Vaccine Booster Shots among Urban Employees: Evidence from a Megacity H in Eastern China. Int. J. Environ. Res. Public Health 2022, 19, 2300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wei, B.; Lin, H.; Wang, Y.; Chai, S.; Liu, W. Nursing students’ attitudes, knowledge and willingness of to receive the coronavirus disease vaccine: A cross-sectional study. Nurse Educ. Pract. 2021, 55, 103148. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, H.E.; Tumambeng, M.Z.; Halawi, M.H.A.; Masmali, E.M.A.; Tashari, T.B.M.; Arishi, F.H.A.; Shadad, R.H.M.; Alfaraj, S.Z.A.; Fathi, S.M.A.; Mahfouz, M.S. COVID-19 vaccine hesitancy prevalence and predictors among the students of Jazan University, Saudi Arabia using the health belief model: A Cross-Sectional Study. Vaccines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Barello, S.; Nania, T.; Dellafiore, F.; Graffigna, G.; Caruso, R. ‘Vaccine hesitancy’among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 2020, 35, 781–783. [Google Scholar] [CrossRef]
- Shah, A.; Marks, P.W.; Hahn, S.M. Unwavering regulatory safeguards for COVID-19 vaccines. Jama 2020, 324, 931–932. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Ball, P.; Maxmen, A. The epic battle against coronavirus misinformation and conspiracy theories. Nature 2020, 581, 371–375. [Google Scholar] [CrossRef]
- Islam, M.S.; Sarkar, T.; Khan, S.H.; Kamal, A.-H.M.; Hasan, S.M.; Kabir, A.; Yeasmin, D.; Islam, M.A.; Chowdhury, K.I.A.; Anwar, K.S. COVID-19–related infodemic and its impact on public health: A global social media analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1621. [Google Scholar] [CrossRef]
- Johnson, N.F.; Velásquez, N.; Restrepo, N.J.; Leahy, R.; Gabriel, N.; El Oud, S.; Zheng, M.; Manrique, P.; Wuchty, S.; Lupu, Y. The online competition between pro-and anti-vaccination views. Nature 2020, 582, 230–233. [Google Scholar] [CrossRef]
- Burki, T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit. Health 2020, 2, e504–e505. [Google Scholar] [CrossRef]
- Ball, P. Anti-vaccine movement could undermine efforts to end coronavirus pandemic, researchers warn. Nature 2020, 581, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.J. A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Commun. 2010, 25, 661–669. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total | Willingness to Receive Vaccine Booster Shots | Chi-Square | p-Value | ||
|---|---|---|---|---|---|---|
| n (%) | Intended (83.9%) | Undecided (10.2%) | Unwilling (5.9%) | |||
| Gender | ||||||
| Female | 381 (57.6) | 323 (84.8) | 48 (12.6) | 10 (2.6) | 15.69 | <0.001 |
| Male | 517 (42.4) | 430 (83.2) | 44 (8.5) | 43 (8.3) | ||
| Age group | ||||||
| 18 and below | 94 (10.5) | 77 (81.9) | 13 (13.8) | 4 (4.3) | 10.39 | 0.109 |
| 19–30 | 676 (75.3) | 559 (82.7) | 73 (10.8) | 44 (6.5) | ||
| 31–40 | 93 (10.4) | 87 (93.5) | 2 (2.2) | 4 (4.3) | ||
| Above 40 | 35 (3.9) | 30 (85.7) | 4 (11.4) | 1 (2.9) | ||
| Living area | ||||||
| Urban | 715 (79.6) | 604 (84.5) | 68 (9.5) | 43 (6.0) | 2.08 | 0.353 |
| Rural | 183 (20.4) | 149 (81.4) | 24 (13.1) | 10 (5.5) | ||
| Educational background | ||||||
| Junior high school and below | 49 (5.5) | 42 (85.7) | 4 (8.2) | 3 (6.1) | 34.22 | <0.001 |
| High school | 131 (14.6) | 104 (79.4) | 17 (13.0) | 10 (7.6) | ||
| Associate college | 230 (25.6) | 206 (89.6) | 11 (4.8) | 13 (5.7) | ||
| Bachelor’s degree | 387 (43.1) | 333 (86.0) | 36 (9.3) | 18 (4.7) | ||
| Master’s degree and above | 101 (11.2) | 68 (67.3) | 24 (23.8) | 9 (8.9) | ||
| Monthly income (yuan) | ||||||
| Under 5000 | 354 (39.4) | 295 (83.3) | 43 (12.1) | 16 (4.5) | 6.72 | 0.347 |
| 5000–8000 | 306 (34.1) | 262 (85.6) | 23 (7.5) | 21 (6.9) | ||
| 8000–12,000 | 175 (19.5) | 146 (83.4) | 17 (9.7) | 12 (6.9) | ||
| Over 12,000 | 63 (7.0) | 50 (79.4) | 9 (14.3) | 4 (6.3) | ||
| Occupation | ||||||
| Medical personnel | 57 (6.3) | 53 (93.0) | 2 (3.5) | 2 (3.5) | 38.3 | <0.001 |
| Civil Service | 132 (14.7) | 111 (84.1) | 10 (7.6) | 11 (8.3) | ||
| Service industry personnel | 162 (18.0) | 139 (85.8) | 6 (3.7) | 17 (10.5) | ||
| Other corporate employees | 242 (26.9) | 200 (82.6) | 32 (13.2) | 10 (4.1) | ||
| Teachers | 39 (4.3) | 34 (87.2) | 1 (2.6) | 4 (10.3) | ||
| Students | 221 (24.6) | 179 (81.0) | 36 (16.3) | 6 (2.7) | ||
| Farmers | 23 (2.6) | 20 (87.0) | 2 (8.7) | 1 (4.3) | ||
| Others | 22 (2.4) | 17 (77.3) | 3 (13.6) | 2 (9.1) | ||
| Risk level of the area | ||||||
| Low Risk | 778 (86.6) | 657 (84.4) | 85 (10.9) | 36 (4.6) | 20.41 | <0.001 |
| Medium Risk | 101 (11.2) | 79 (78.2) | 7 (6.9) | 15 (14.9) | ||
| High Risk | 19 (2.1) | 17 (89.5) | 0 (0.0) | 2 (10.5) | ||
| Medical insurance | ||||||
| Yes | 813 (90.5) | 687 (84.5) | 78 (9.6) | 48 (5.9) | 3.98 | 0.136 |
| No | 85 (9.5) | 66 (77.6) | 14 (16.5) | 5 (5.9) | ||
| Variables | Total | Willingness to Get COVID-19 Vaccine Boosters | Chi-Square | p-Value | ||
|---|---|---|---|---|---|---|
| n (%) | Intended (83.9%) | Undecided (10.2%) | Unwilling (5.9%) | |||
| Type of vaccines (Classified by the times of injections) | ||||||
| 1 injection | 23 (2.6) | 15 (65.2) | 3 (13.0) | 5 (21.7) | 18.18 | 0.001 |
| 2 injections | 490 (54.6) | 399 (81.4) | 59 (12.0) | 32 (6.5) | ||
| 3 injections | 385 (42.9) | 339 (88.1) | 30 (7.8) | 16 (4.2) | ||
| Manufacturer of vaccines | ||||||
| Wuhan Institute of Biological Products in Wuhan, China | 88 (9.8) | 77 (87.5) | 8 (9.1) | 3 (3.4) | 27.11 | 0.007 |
| Beijing Institute of Biological Products Co., Ltd. in Beijing, China | 121 (13.5) | 102 (84.3) | 10 (8.3) | 9 (7.4) | ||
| Sinovac Biotech Co., Ltd. in Beijing, China | 514 (57.2) | 445 (86.6) | 43 (8.4) | 26 (5.1) | ||
| Tianjin Cansino Biotechnology Inc. in Tianjin, China | 41 (4.6) | 30 (73.2) | 5 (12.2) | 6 (14.6) | ||
| Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. in Anhui, China | 40 (4.5) | 27 (67.5) | 10 (25.0) | 3 (7.5) | ||
| Other manufacturers | 16 (1.8) | 11 (68.8) | 4 (25.0) | 1 (6.3) | ||
| No knowledge of the manufacturer | 78 (8.7) | 61 (78.2) | 12 (15.4) | 5 (6.4) | ||
| Perceived effects from the primary series | ||||||
| Very high | 392 (43.7) | 373 (95.2) | 9 (2.3) | 10 (2.6) | 259.3 | <0.001 |
| High | 372 (41.4) | 323 (86.8) | 37 (9.9) | 12 (3.2) | ||
| Moderate | 115 (12.8) | 50 (43.5) | 44 (38.3) | 21 (18.3) | ||
| Low | 13 (1.4) | 4 (30.8) | 2 (15.4) | 7 (53.8) | ||
| Very low | 6 (0.7) | 3 (50.0) | 0 (0.0) | 3 (50.0) | ||
| Friends’ willingness to receive booster shots | 697.0 | |||||
| Very high | 452 (50.3) | 438 (96.9) | 7 (1.5) | 7 (1.5) | ||
| High | 296 (33.0) | 271 (91.6) | 18 (6.1) | 7 (2.4) | <0.001 | |
| Moderate | 113 (12.6) | 35 (31.0) | 66 (58.4) | 12 (10.6) | ||
| Low | 19 (2.1) | 8 (42.1) | 1 (5.3) | 10 (52.6) | ||
| Very low | 18 (2.0) | 1 (5.6) | 0 (0.0) | 17 (94.4) | ||
| Family members’ willingness to receive booster shots | ||||||
| Very high | 505 (56.2) | 492 (97.4) | 4 (0.8) | 9 (1.8) | 528.9 | <0.001 |
| High | 249 (27.7) | 215 (86.3) | 27 (10.8) | 7 (2.8) | ||
| Moderate | 110 (12.2) | 39 (35.5) | 55 (50.0) | 16 (14.5) | ||
| Low | 20 (2.2) | 7 (35.0) | 5 (25.0) | 8 (40.0) | ||
| Very low | 14 (1.6) | 0 (0.0) | 1 (7.1) | 13 (92.9) | ||
| Reasons for Receiving Booster Shots | n (%) |
|---|---|
| Supporting vaccination policy in China | 282 (48.9) |
| Vaccination required by workplace or school | 73 (12.7) |
| Further enhancing the protective effect of the COVID-19 vaccine | 155 (26.9) |
| Fears of contracting a mutant strain of the coronavirus despite vaccination | 57 (9.9) |
| Chose to receive the booster vaccination because of others’ vaccination | 10 (1.7) |
| Items | Cronbach’s α | AVE 1 | CR 2 |
|---|---|---|---|
| Perceived Severity | 0.822 | 0.565 | 0.834 |
| Perceived Susceptibility | 0.705 | 0.553 | 0.710 |
| Perceived Benefits | 0.876 | 0.641 | 0.877 |
| Perceived Barriers | 0.917 | 0.742 | 0.919 |
| Self-Efficacy | 0.832 | 0.621 | 0.831 |
| Cues to Action | 0.836 | 0.631 | 0.837 |
| Perceived Severity | Perceived Susceptibility | Perceived Benefits | Perceived Barriers | Self-Efficacy | Cues to Action | |
|---|---|---|---|---|---|---|
| Perceived Severity | 0.752 1 | |||||
| Perceived Susceptibility | 0.385 | 0.744 | ||||
| Perceived Benefits | 0.284 | 0.352 | 0.801 | |||
| Perceived Barriers | 0.225 | 0.104 | −0.213 | 0.861 | ||
| Self-Efficacy | 0.305 | 0.315 | 0.655 | −0.101 | 0.788 | |
| Cues to Action | 0.302 | 0.299 | 0.670 | −0.107 | 0.723 | 0.794 |
| Paths | C.R. 1 | Unstandardized Path Coefficients 2 | Standardized Path Coefficients 2 | p-Value |
|---|---|---|---|---|
| Perceived Severity → Booster Vaccination willingness | −0.561 | −0.031 | −0.023 | 0.575 |
| Perceived Susceptibility → Booster Vaccination willingness | −2.207 | −0.125 | −0.109 | 0.027 |
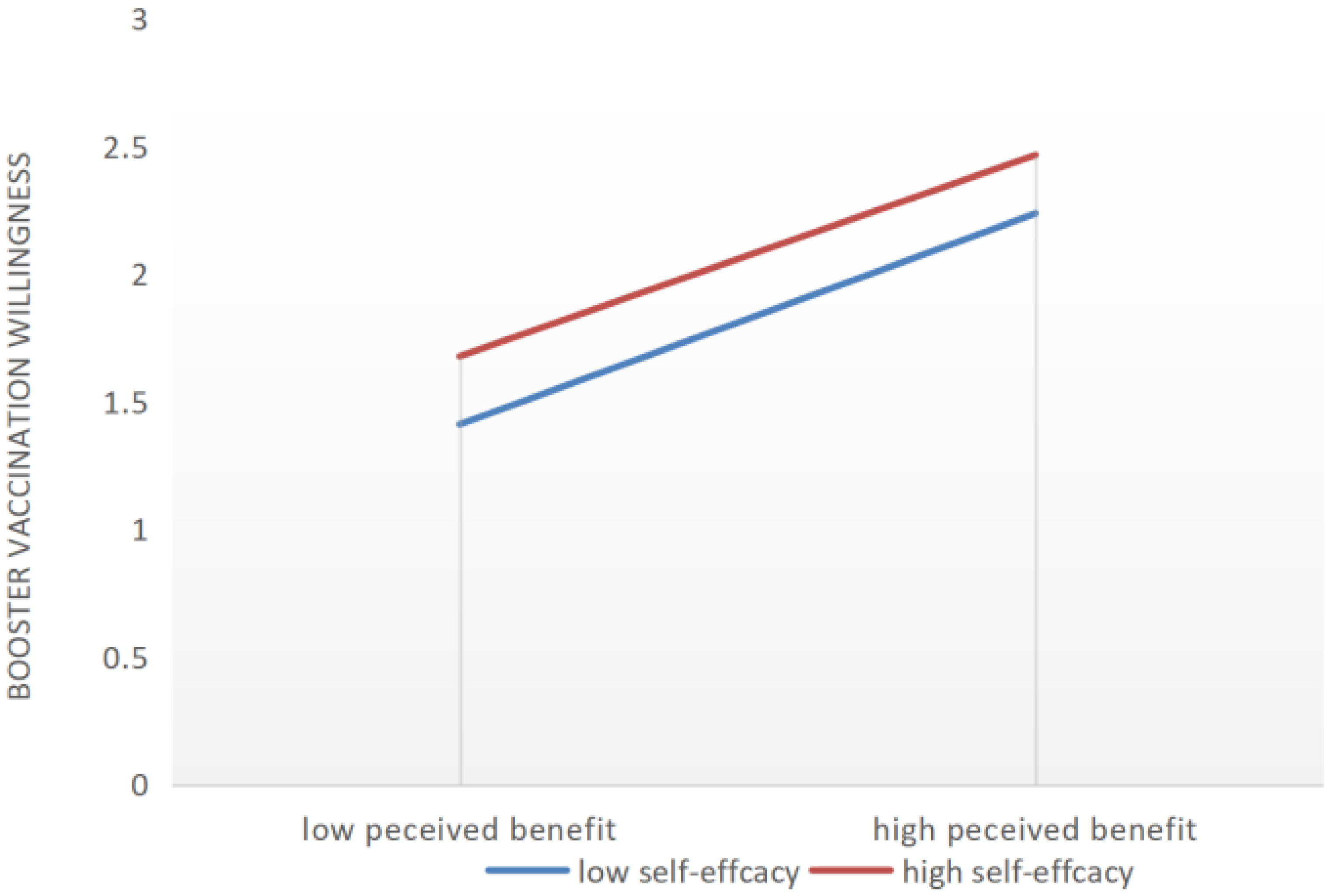
| Perceived Benefit → Booster Vaccination willingness | 2.102 | 0.233 | 0.148 | 0.036 |
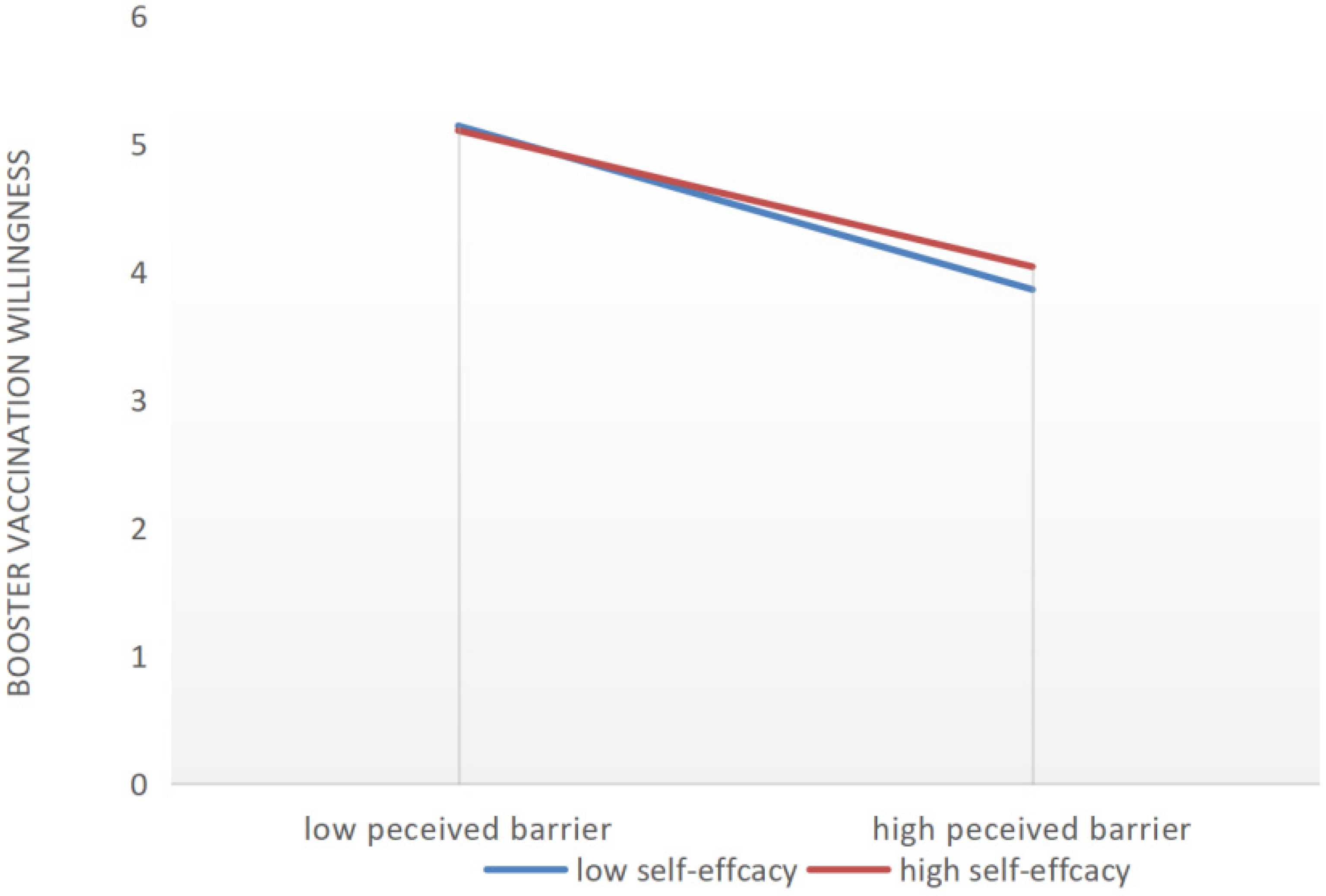
| Perceived Barriers → Booster Vaccination willingness | −4.053 | −0.139 | −0.151 | <0.001 |
| Cues to Action → Booster Vaccination willingness | 2.977 | 0.438 | 0.308 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Liu, Z.; Gong, L.; Kong, Y.; Liu, H.; Wei, C.; Wu, X.; Zhu, Q.; Guo, Y. Exploring the Willingness of the COVID-19 Vaccine Booster Shots in China Using the Health Belief Model: Web-Based Online Cross-Sectional Study. Vaccines 2022, 10, 1336. https://doi.org/10.3390/vaccines10081336
Hu D, Liu Z, Gong L, Kong Y, Liu H, Wei C, Wu X, Zhu Q, Guo Y. Exploring the Willingness of the COVID-19 Vaccine Booster Shots in China Using the Health Belief Model: Web-Based Online Cross-Sectional Study. Vaccines. 2022; 10(8):1336. https://doi.org/10.3390/vaccines10081336
Chicago/Turabian StyleHu, Dehua, Zhisheng Liu, Liyue Gong, Yi Kong, Hao Liu, Caiping Wei, Xusheng Wu, Qizhen Zhu, and Yi Guo. 2022. "Exploring the Willingness of the COVID-19 Vaccine Booster Shots in China Using the Health Belief Model: Web-Based Online Cross-Sectional Study" Vaccines 10, no. 8: 1336. https://doi.org/10.3390/vaccines10081336
APA StyleHu, D., Liu, Z., Gong, L., Kong, Y., Liu, H., Wei, C., Wu, X., Zhu, Q., & Guo, Y. (2022). Exploring the Willingness of the COVID-19 Vaccine Booster Shots in China Using the Health Belief Model: Web-Based Online Cross-Sectional Study. Vaccines, 10(8), 1336. https://doi.org/10.3390/vaccines10081336






