Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels
Abstract
:1. Introduction
2. Materials and Methods
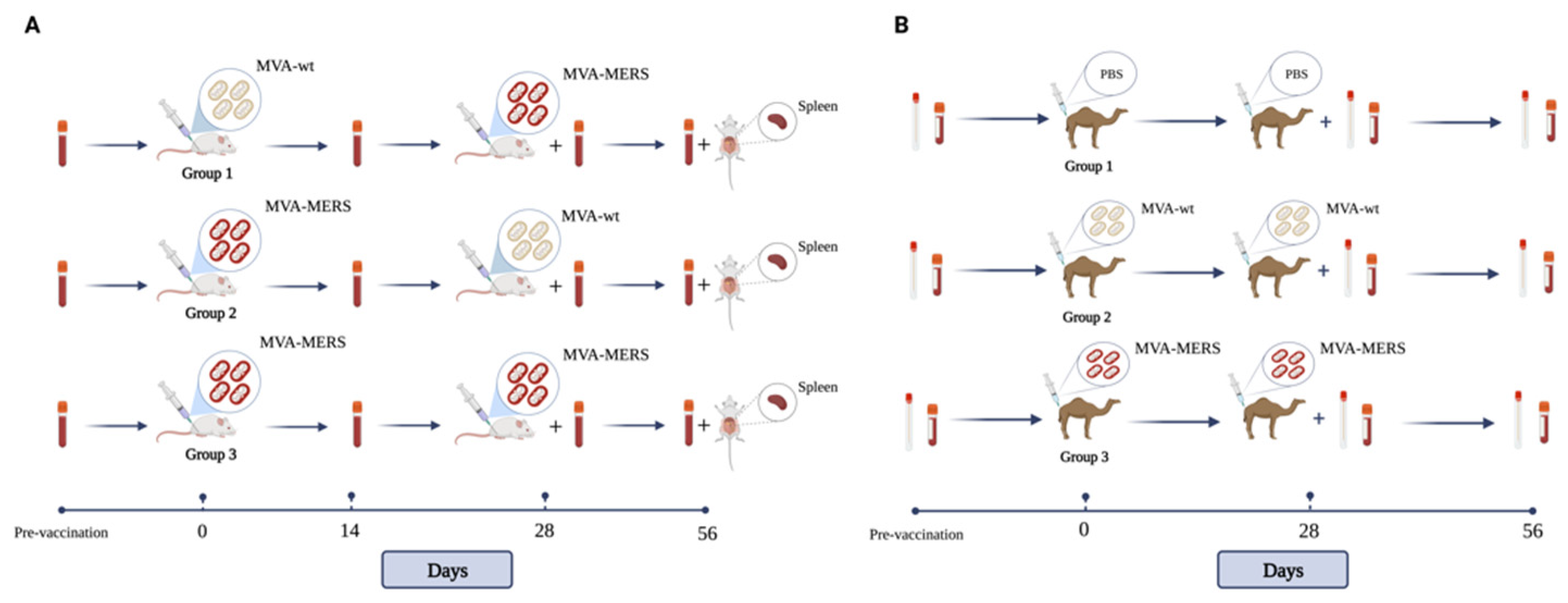
2.1. Vaccine, Immunisation, and Samples’ Collection
2.2. ELISA
2.3. Mouse Ex Vivo IFN-γ ELISpot
2.4. MERS Pseudotyped Viral Particles (MERSpp) Neutralisation Assay
2.5. Data Analysis
3. Results
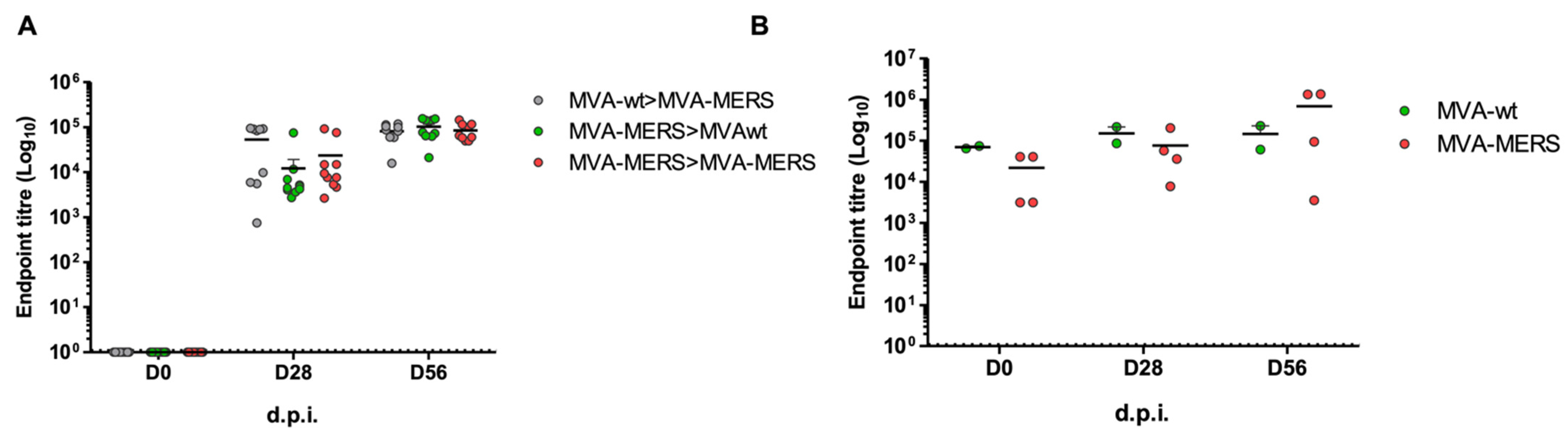
3.1. MVA-MERS Immunogenicity in Mouse Models
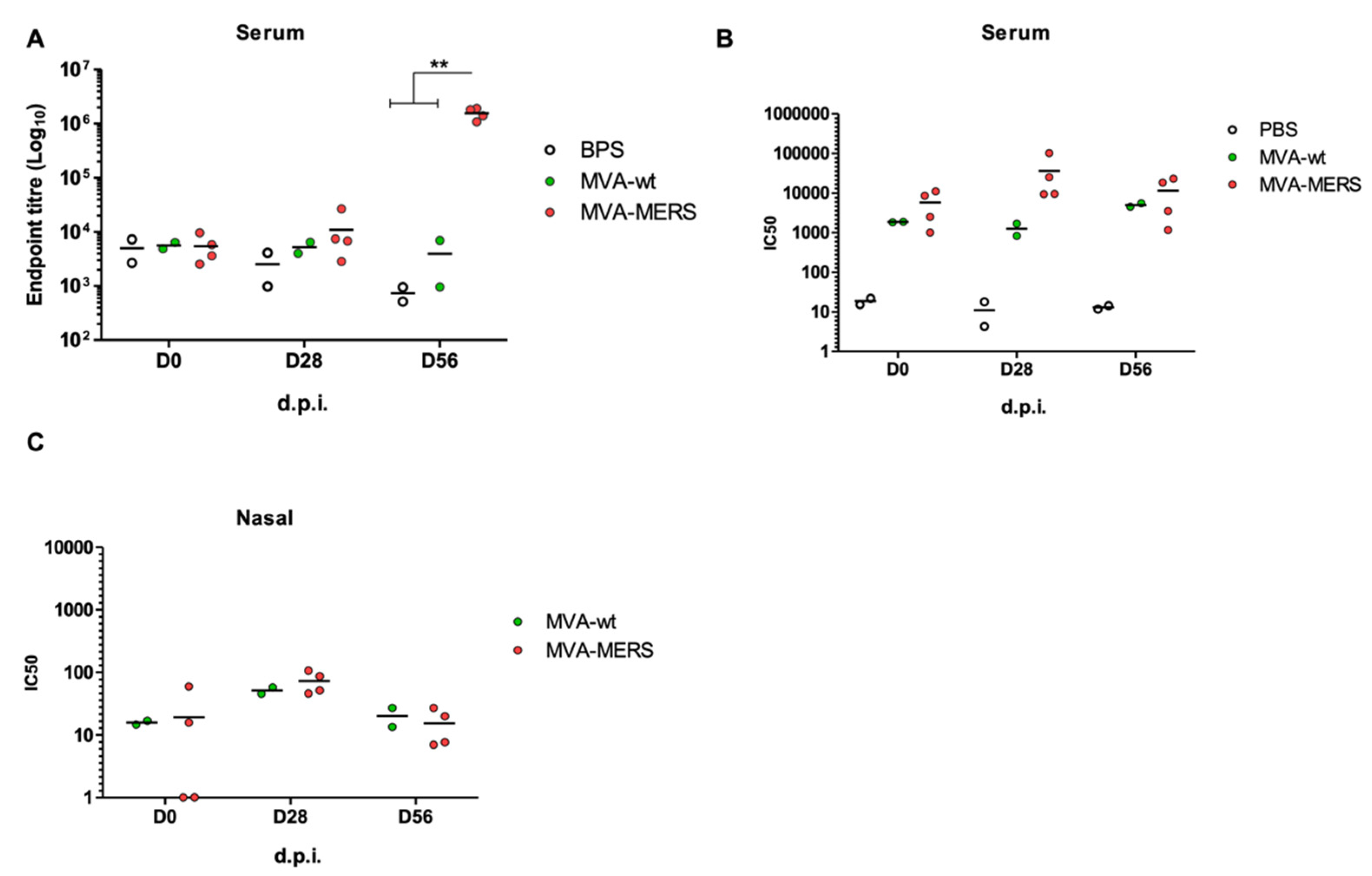
3.2. MVA-MERS Immunogenicity in Dromedary Camels
3.3. Anti-MVA Immune Responses in Vaccinated Mice and Camels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochstein-Mintzel, V.; Huber, H.C.; Stickl, H. Virulence and immunogenicity of a modified vaccinia virus (strain MVA) (author’s transl). Z. Immun. Exp. Klin. Immunol. 1972, 144, 104–156. [Google Scholar]
- Mayr, A.; Stickl, H.; Müller, H.K.; Danner, K.; Singer, H. The smallpox vaccination strain MVA: Marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl). Zent. Bakteriol. Parasitenkd. Infekt. Hyg. Erste Abt. Orig. Reihe B Hyg. Betr. Prav. Med. 1978, 167, 375–390. [Google Scholar]
- Volz, A.; Sutter, G. Protective efficacy of Modified Vaccinia virus Ankara in preclinical studies. Vaccine 2013, 31, 4235–4240. [Google Scholar] [CrossRef]
- Gilbert, S.C. Clinical development of Modified Vaccinia virus Ankara vaccines. Vaccine 2013, 31, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Von Krempelhuber, A.; Vollmar, J.; Pokorny, R.; Rapp, P.; Wulff, N.; Petzold, B.; Handley, A.; Mateo, L.; Siersbol, H.; Kollaritsch, H.; et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 2010, 28, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- SMALLPOX MVA-BN®. Bavarian Nordic. Available online: https://www.bavarian-nordic.com/pipeline/mva-bn.aspx (accessed on 22 May 2022).
- Orubu, T.; Alharbi, N.K.; Lambe, T.; Gilbert, S.; Cottingham, M.G. Expression and Cellular Immunogenicity of a Transgenic Antigen Driven by Endogenous Poxviral Early Promoters at Their Authentic Loci in MVA. PLoS ONE 2012, 7, e40167. [Google Scholar] [CrossRef]
- Garcés, J.; Masternak, K.; Kunz, B.; Wittek, R. Reactivation of transcription from a vaccinia virus early promoter late in infection. J. Virol. 1993, 67, 5394–5401. [Google Scholar] [CrossRef]
- Wennier, S.T.; Brinkmann, K.; Steinhäußer, C.; Mayländer, N.; Mnich, C.; Wielert, U.; Dirmeier, U.; Hausmann, J.; Chaplin, P.; Steigerwald, R. A Novel Naturally Occurring Tandem Promoter in Modified Vaccinia Virus Ankara Drives Very Early Gene Expression and Potent Immune Responses. PLoS ONE 2013, 8, e73511. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Sisler, J.R.; Moss, B. Compact, Synthetic, Vaccinia Virus Early/Late Promoter for Protein Expression. BioTechniques 1997, 23, 1094–1097. [Google Scholar] [CrossRef]
- Hammond, J.M.; Oke, P.G.; Coupar, B.E. A synthetic vaccinia virus promoter with enhanced early and late activity. J. Virol. Methods 1997, 66, 135–138. [Google Scholar] [CrossRef]
- Davison, A.J.; Moss, B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990, 18, 4285. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Moss, B. Structure of vaccinia virus early promoters. J. Mol. Biol. 1989, 210, 749–769. [Google Scholar] [CrossRef]
- Wang, Z.; Martinez, J.; Zhou, W.; La Rosa, C.; Srivastava, T.; Dasgupta, A.; Rawal, R.; Li, Z.; Britt, W.J.; Diamond, D. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 2010, 28, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Embry, A.; Meng, X.; Cantwell, A.; Dube, P.H.; Xiang, Y. Enhancement of immune response to an antigen delivered by vaccinia virus by displaying the antigen on the surface of intracellular mature virion. Vaccine 2011, 29, 5331–5339. [Google Scholar] [CrossRef]
- Alharbi, N.K. Poxviral promoters for improving the immunogenicity of MVA delivered vaccines. Hum. Vaccines Immunother. 2019, 15, 203–209. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Spencer, A.J.; Salman, A.M.; Tully, C.M.; Chinnakannan, S.K.; Lambe, T.; Yamaguchi, Y.; Morris, S.J.; Orubu, T.; Draper, S.J.; et al. Enhancing cellular immunogenicity of MVA-vectored vaccines by utilizing the F11L endogenous promoter. Vaccine 2016, 34, 49–55. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Spencer, A.; Hill, A.V.S.; Gilbert, S.C. Deletion of Fifteen Open Reading Frames from Modified Vaccinia Virus Ankara Fails to Improve Immunogenicity. PLoS ONE 2015, 10, e0128626. [Google Scholar] [CrossRef]
- Delaloye, J.; Roger, T.; Steiner-Tardivel, Q.-G.; Le Roy, D.; Reymond, M.K.; Akira, S.; Petrilli, V.; Gomez, C.E.; Perdiguero, B.; Tschopp, J.; et al. Innate Immune Sensing of Modified Vaccinia Virus Ankara (MVA) Is Mediated by TLR2-TLR6, MDA-5 and the NALP3 Inflammasome. PLoS Pathog. 2009, 5, e1000480. [Google Scholar] [CrossRef]
- Samuelsson, C.; Hausmann, J.; Lauterbach, H.; Schmidt, M.; Akira, S.; Wagner, H.; Chaplin, P.; Suter, M.; O’Keeffe, M.; Hochrein, H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 2008, 118, 1776–1784. [Google Scholar] [CrossRef]
- Lousberg, E.; Diener, K.R.; Brown, M.P.; Hayball, J.D. Innate immune recognition of poxviral vaccine vectors. Expert Rev. Vaccines 2011, 10, 1435–1449. [Google Scholar] [CrossRef]
- Lehmann, M.H.; Kastenmuller, W.; Kandemir, J.D.; Brandt, F.; Suezer, Y.; Sutter, G. Modified Vaccinia Virus Ankara Triggers Chemotaxis of Monocytes and Early Respiratory Immigration of Leukocytes by Induction of CCL2 Expression. J. Virol. 2009, 83, 2540–2552. [Google Scholar] [CrossRef] [PubMed]
- Drexler, I.; Staib, C.; Sutter, G. Modified vaccinia virus Ankara as antigen delivery system: How can we best use its potential? Curr. Opin. Biotechnol. 2004, 15, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gordo, E.; Podgorski, I.I.; Downes, N.; Alemany, R. Circumventing Antivector Immunity: Potential Use of Nonhuman Adenoviral Vectors. Hum. Gene Ther. 2014, 25, 285–300. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Padron-Regalado, E.; Thompson, C.P.; Kupke, A.; Wells, D.; Sloan, M.; Grehan, K.; Temperton, N.; Lambe, T.; Warimwe, G.; et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 2017, 35, 3780–3788. [Google Scholar] [CrossRef]
- Adney, D.R.; Wang, L.; van Doremalen, N.; Shi, W.; Zhang, Y.; Kong, W.-P.; Miller, M.R.; Bushmaker, T.; Scott, D.; de Wit, E.; et al. Efficacy of an Adjuvanted Middle East Respiratory Syndrome Coronavirus Spike Protein Vaccine in Dromedary Camels and Alpacas. Viruses 2019, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Volz, A.; Kupke, A.; Song, F.; Jany, S.; Fux, R.; Shams-Eldin, H.; Schmidt, J.; Becker, C.; Eickmann, M.; Becker, S.; et al. Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein. J. Virol. 2015, 89, 8651–8656. [Google Scholar] [CrossRef]
- Hashem, A.M.; Algaissi, A.; Agrawal, A.S.; Al-Amri, S.S.; Alhabbab, R.Y.; Sohrab, S.S.; Almasoud, A.S.; Alharbi, N.K.; Peng, B.-H.; Russell, M.; et al. A Highly Immunogenic, Protective, and Safe Adenovirus-Based Vaccine Expressing Middle East Respiratory Syndrome Coronavirus S1-CD40L Fusion Protein in a Transgenic Human Dipeptidyl Peptidase 4 Mouse Model. J. Infect. Dis. 2019, 220, 1558–1567. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Brand, J.M.A.V.D.; Raj, V.S.; Volz, A.; Wohlsein, P.; Smits, S.L.; Schipper, D.; Bestebroer, T.M.; Okba, N.; Fux, R.; et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016, 351, 77–81. [Google Scholar] [CrossRef]
- Bosaeed, M.; Balkhy, H.H.; Almaziad, S.; Aljami, H.A.; Alhatmi, H.; Alanazi, H.; Alahmadi, M.; Jawhary, A.; Alenazi, M.W.; Almasoud, A.; et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): An open-label, non-randomised, dose-escalation, phase 1b trial. Lancet Microbe 2022, 3, e11–e20. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) (accessed on 28 December 2020).
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.A.B.A.; Reusken, C.B.E.M.; Haagmans, B.L.; Mohran, K.A.; Raj, V.S.; Pas, S.D.; Voermans, J.; Smits, S.L.; Godeke, G.-J.; Al-Hajri, M.M.; et al. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect. Ecol. Epidemiol. 2015, 5, 28305. [Google Scholar] [CrossRef] [PubMed]
- Aljasim, T.; Almasoud, A.; Aljami, H.; Alenazi, M.; Alsagaby, S.; Alsaleh, A.; Alharbi, N. High Rate of Circulating MERS-CoV in Dromedary Camels at Slaughterhouses in Riyadh, 2019. Viruses 2020, 12, 1215. [Google Scholar] [CrossRef] [PubMed]
- Tolah, A.M.; Al Masaudi, S.B.; El-Kafrawy, S.A.; Mirza, A.A.; Harakeh, S.M.; Hassan, A.M.; Alsaadi, M.A.; Alzahrani, A.A.; Alsaaidi, G.A.; Amor, N.M.S.; et al. Cross-sectional prevalence study of MERS-CoV in local and imported dromedary camels in Saudi Arabia, 2016–2018. PLoS ONE 2020, 15, e0232790. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Corman, V.M.; Tolah, A.M.; Al Masaudi, S.B.; Hassan, A.M.; Müller, M.A.; Bleicker, T.; Harakeh, S.M.; Alzahrani, A.A.; Alsaaidi, G.A. Enzootic patterns of Middle East respiratory syndrome coronavirus in imported African and local Arabian dromedary camels: A prospective genomic study. Lancet Planet. Health 2019, 3, e521–e528. [Google Scholar] [CrossRef]
- Alshukairi, A.N.; Zhao, J.; Al-Mozaini, M.A.; Wang, Y.; Dada, A.; Baharoon, S.A.; Alfaraj, S.; Ahmed, W.A.; Enani, M.A.; Elzein, F.E.; et al. Longevity of Middle East Respiratory Syndrome Coronavirus Antibody Responses in Humans, Saudi Arabia. Emerg. Infect. Dis. 2021, 27, 1472–1476. [Google Scholar] [CrossRef]
- Alhabbab, R.Y.; Algaissi, A.; Mahmoud, A.B.; A Alkayyal, A.; Al-Amri, S.; Alfaleh, M.A.; Basabrain, M.; Alsubki, R.A.; Almarshad, I.S.; Alhudaithi, A.M.; et al. MERS-CoV infection elicits long-lasting specific antibody, T and B cell immune responses in recovered individuals. Clin. Infect. Dis. 2022, ciac456. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Qasim, I.; Almasoud, A.; Aljami, H.A.; Alenazi, M.W.; Alhafufi, A.; Aldibasi, O.S.; Hashem, A.M.; Kasem, S.; Albrahim, R.; et al. Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels. Sci. Rep. 2019, 9, 16292. [Google Scholar] [CrossRef]
- Draper, S.J.; Moore, A.; Goodman, A.L.; Long, C.A.; Holder, A.; Gilbert, S.; Hill, F.; Hill, A.V.S. Effective induction of high-titer antibodies by viral vector vaccines. Nat. Med. 2008, 14, 819–821. [Google Scholar] [CrossRef]
- Falzarano, D.; Kamissoko, B.; de Wit, E.; Maïga, O.; Cronin, J.; Samaké, K.; Traoré, A.; Milne-Price, S.; Munster, V.; Sogoba, N.; et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health 2017, 3, 41–43. [Google Scholar] [CrossRef]
- Cottingham, M.G.; Andersen, R.F.; Spencer, A.; Saurya, S.; Furze, J.; Hill, A.V.S.; Gilbert, S. Recombination-Mediated Genetic Engineering of a Bacterial Artificial Chromosome Clone of Modified Vaccinia virus Ankara (MVA). PLoS ONE 2008, 3, e1638. [Google Scholar] [CrossRef] [PubMed]
- Grehan, K.; Ferrara, F.; Temperton, N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX 2015, 2, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Almasaud, A.; Alharbi, N.K.; Hashem, A.M. Generation of MERS-CoV Pseudotyped Viral Particles for the Evaluation of Neutralizing Antibodies in Mammalian Sera. Methods Mol. Biol. 2020, 2099, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.E.; Timares, L.; Matthews, Q.L. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev. Vaccines 2009, 8, 761–777. [Google Scholar] [CrossRef]
- Omrani, A.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): Animal to human interaction. Pathog. Glob. Health 2015, 109, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kasem, S.; Qasim, I.; Al-Hufofi, A.; Hashim, O.; Alkarar, A.; Abu-Obeida, A.; Gaafer, A.; Elfadil, A.; Zaki, A.; Al-Romaihi, A.; et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J. Infect. Public Health 2017, 11, 331–338. [Google Scholar] [CrossRef]
- Reusken, C.B.; Haagmans, B.L.; Müller, M.A.; Gutierrez, C.; Godeke, G.-J.; Meyer, B.; Muth, D.; Raj, V.S.; Vries, L.S.-D.; Corman, V.M.; et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect. Dis. 2013, 13, 859–866. [Google Scholar] [CrossRef]
- Reusken, C.B.; Ababneh, M.; Raj, V.S.; Meyer, B.; Eljarah, A.; Abutarbush, S.; Godeke, G.J.; Bestebroer, T.M.; Zutt, I.; Müller, M.A.; et al. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Eurosurveillance 2013, 18, 20662. [Google Scholar] [CrossRef]
- Saqib, M.; Sieberg, A.; Hussain, M.H.; Mansoor, M.K.; Zohaib, A.; Lattwein, E.; Müller, M.A.; Drosten, C.; Corman, V.M. Serologic Evidence for MERS-CoV Infection in Dromedary Camels, Punjab, Pakistan, 2012–2015. Emerg. Infect. Dis. 2017, 23, 550–551. [Google Scholar] [CrossRef]
- Müller, M.A.; Corman, V.M.; Jores, J.; Meyer, B.; Younan, M.; Liljander, A.; Bosch, B.-J.; Lattwein, E.; Hilali, M.A.; Musa, B.E.; et al. MERS Coronavirus Neutralizing Antibodies in Camels, Eastern Africa, 1983–1997. Emerg. Infect. Dis. 2014, 20, 2093–2095. [Google Scholar] [CrossRef]
- Wernery, U.; Corman, V.M.; Wong, E.Y.; Tsang, A.K.; Muth, D.; Lau, S.K.P.; Khazanehdari, K.; Zirkel, F.; Ali, M.; Nagy, P.; et al. Acute Middle East Respiratory Syndrome Coronavirus Infection in Livestock Dromedaries, Dubai, 2014. Emerg. Infect. Dis. 2015, 21, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Liu, Y.V.; Mu, H.; Taylor, J.K.; Massare, M.; Flyer, D.C.; Glenn, G.M.; Smith, G.E.; Frieman, M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014, 32, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.S.; Abbas, A.T.; Siddiq, L.A.; Alghamdi, A.; Sanki, M.A.; Al-Muhanna, M.K.; Alhabbab, R.Y.; Azhar, E.; Li, X.; Hashem, A.M. Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein. Sci. Rep. 2017, 7, 44875. [Google Scholar] [CrossRef]
- Ma, C.; Wang, L.; Tao, X.; Zhang, N.; Yang, Y.; Tseng, C.-T.K.; Li, F.; Zhou, Y.; Jiang, S.; Du, L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design. Vaccine 2014, 32, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, K.; Falzarano, D.; Reuschel, E.L.; Tingey, C.; Flingai, S.; Villarreal, D.O.; Wise, M.; Patel, A.; Izmirly, A.; Aljuaid, A.; et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015, 7, 301ra132. [Google Scholar] [CrossRef]
- Munster, V.J.; Wells, D.; Lambe, T.; Wright, D.; Fischer, R.J.; Bushmaker, T.; Saturday, G.; Van Doremalen, N.; Gilbert, S.C.; De Wit, E.; et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2017, 2, 28. [Google Scholar] [CrossRef]
- Tai, W.; Wang, Y.; Fett, C.A.; Zhao, G.; Li, F.; Perlman, S.; Jiang, S.; Zhou, Y.; Du, L. Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants. J. Virol. 2017, 91, e01651-16. [Google Scholar] [CrossRef]
- Koch, T.; Dahlke, C.; Fathi, A.; Kupke, A.; Krähling, V.; A Okba, N.M.; Halwe, S.; Rohde, C.; Eickmann, M.; Volz, A.; et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: An open-label, phase 1 trial. Lancet Infect. Dis. 2020, 20, 827–838. [Google Scholar] [CrossRef]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.-D.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef]
- Liu, R.-Q.; Ge, J.-Y.; Wang, J.-L.; Shao, Y.; Zhang, H.-L.; Wen, Z.-Y.; Bu, Z.-G. Newcastle disease virus-based MERS-CoV candidate vaccine elicits high-level and lasting neutralizing antibodies in Bactrian camels. J. Integr. Agric. 2017, 16, 2264–2273. [Google Scholar] [CrossRef]
- Wirblich, C.; Coleman, C.M.; Kurup, D.; Abraham, T.S.; Bernbaum, J.G.; Jahrling, P.B.; Hensley, L.E.; Johnson, R.F.; Frieman, M.B.; Schnell, M.J. One-Health: A Safe, Efficient, Dual-Use Vaccine for Humans and Animals against Middle East Respiratory Syndrome Coronavirus and Rabies Virus. J. Virol. 2017, 91, e02040-16. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, N.K.; Aljamaan, F.; Aljami, H.A.; Alenazi, M.W.; Albalawi, H.; Almasoud, A.; Alharthi, F.J.; Azhar, E.I.; Barhoumi, T.; Bosaeed, M.; et al. Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels. Vaccines 2022, 10, 1330. https://doi.org/10.3390/vaccines10081330
Alharbi NK, Aljamaan F, Aljami HA, Alenazi MW, Albalawi H, Almasoud A, Alharthi FJ, Azhar EI, Barhoumi T, Bosaeed M, et al. Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels. Vaccines. 2022; 10(8):1330. https://doi.org/10.3390/vaccines10081330
Chicago/Turabian StyleAlharbi, Naif Khalaf, Fahad Aljamaan, Haya A. Aljami, Mohammed W. Alenazi, Hind Albalawi, Abdulrahman Almasoud, Fatima J. Alharthi, Esam I. Azhar, Tlili Barhoumi, Mohammad Bosaeed, and et al. 2022. "Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels" Vaccines 10, no. 8: 1330. https://doi.org/10.3390/vaccines10081330
APA StyleAlharbi, N. K., Aljamaan, F., Aljami, H. A., Alenazi, M. W., Albalawi, H., Almasoud, A., Alharthi, F. J., Azhar, E. I., Barhoumi, T., Bosaeed, M., Gilbert, S. C., & Hashem, A. M. (2022). Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels. Vaccines, 10(8), 1330. https://doi.org/10.3390/vaccines10081330







