A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats
Abstract
1. Introduction
2. Materials and Methods
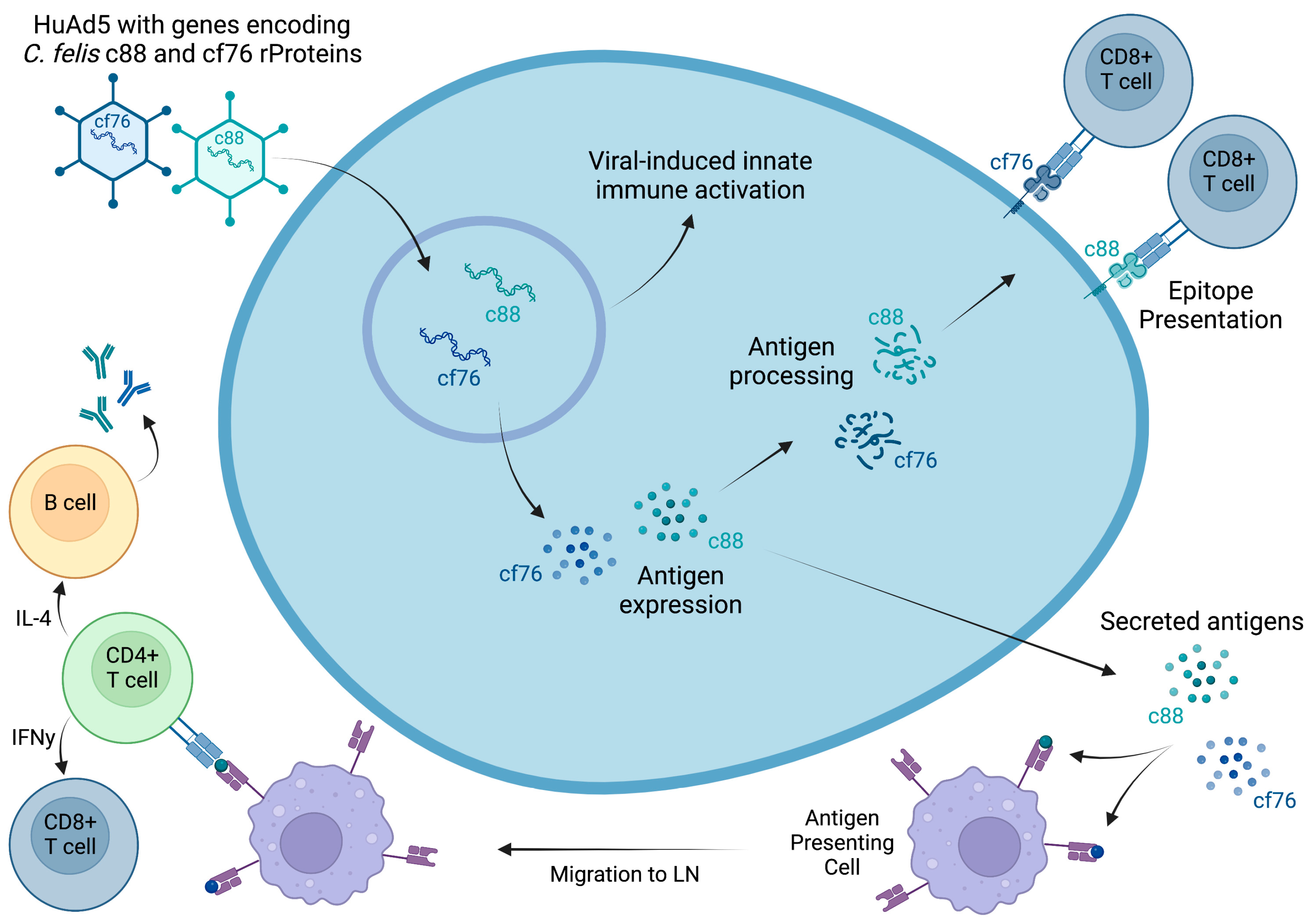
2.1. Construction of Ad-C88/CF76 Overexpression Vector and Generation of Adenovirus
2.2. Animals
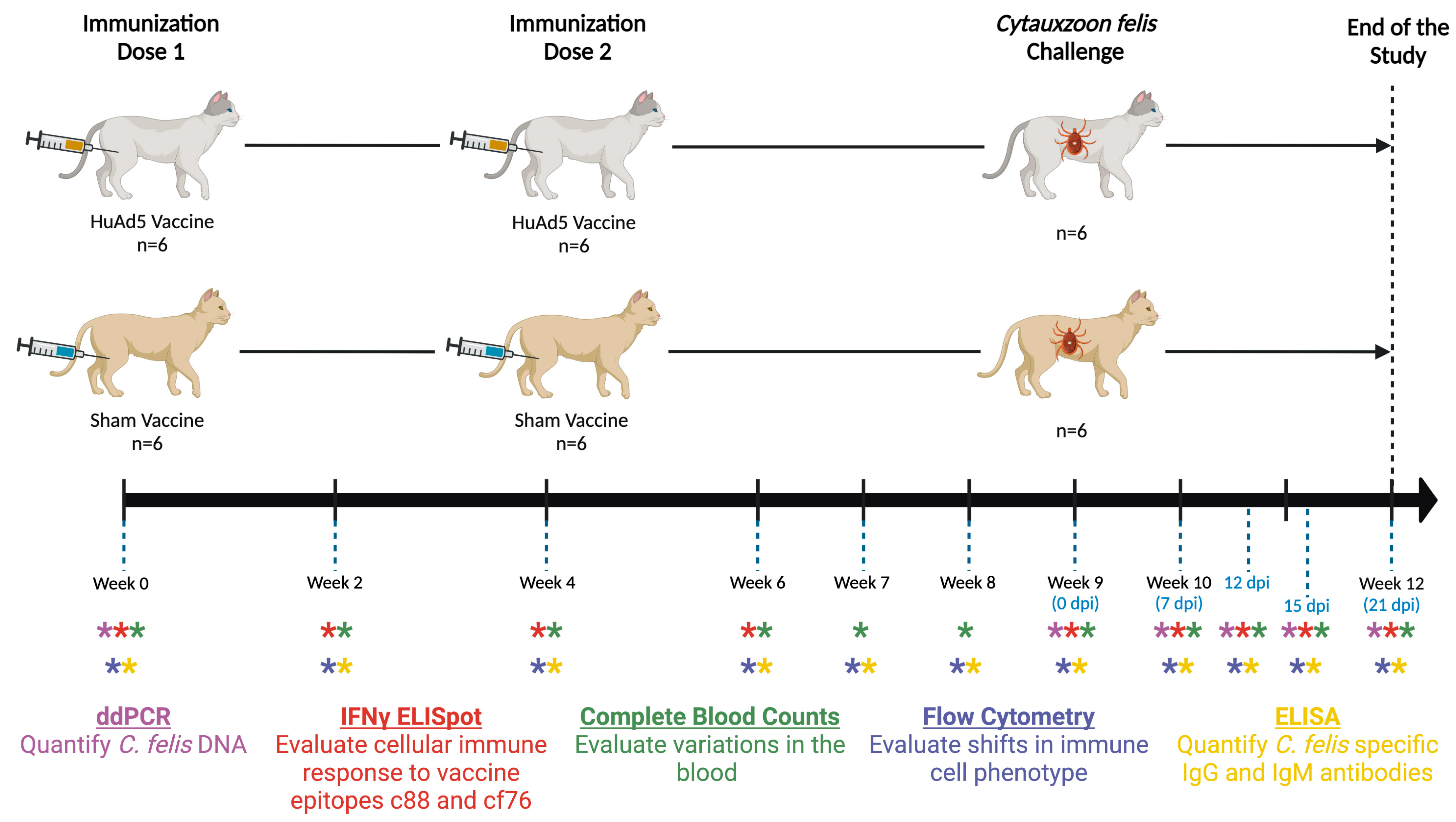
2.3. Immunization and Challenge of Cats
2.4. Sampling
2.5. Flow Cytometry
2.6. ELISpot Assay
2.7. IgM and IgG ELISAs
2.8. Droplet Digital PCR (ddPCR)
2.9. Clinical Scoring
2.10. Histopathology
2.11. Quantification of Large Granular Lymphocytes
2.12. Statistical Analysis
3. Results
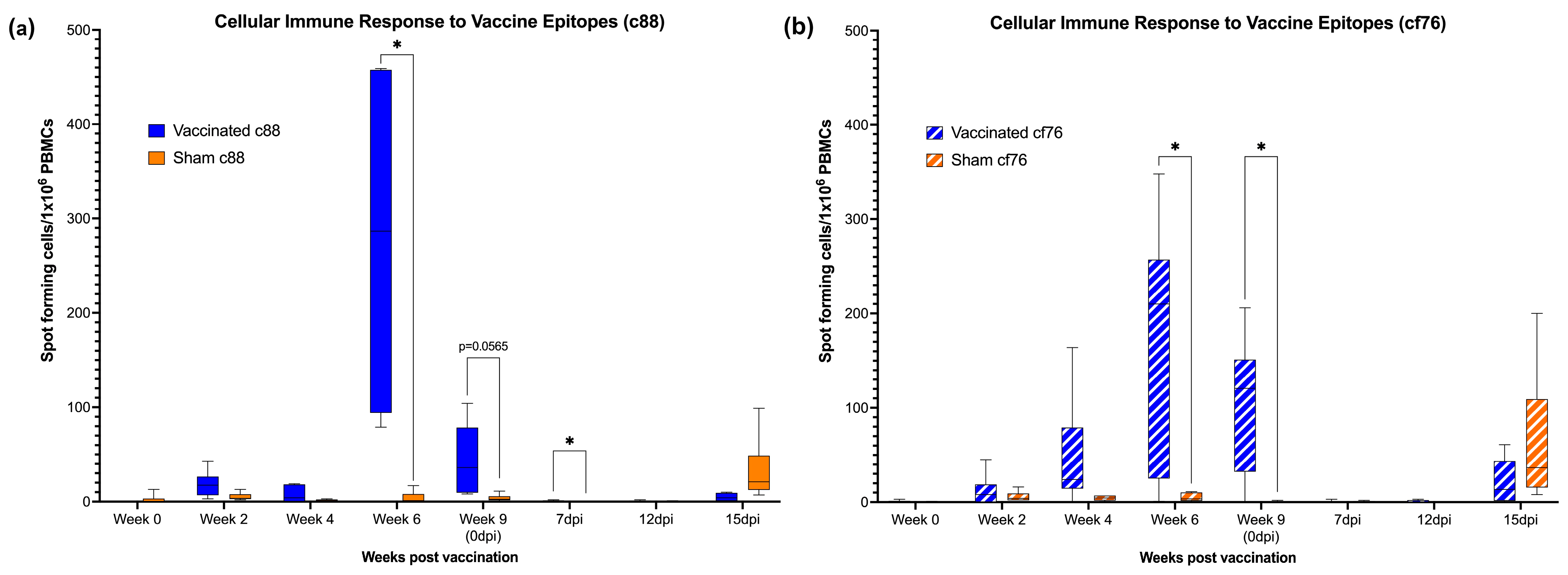
3.1. Pronounced Cell-Mediated Immune Response to Vaccination
3.2. Increased Anti-c88 IgG Levels in Immunized Cats
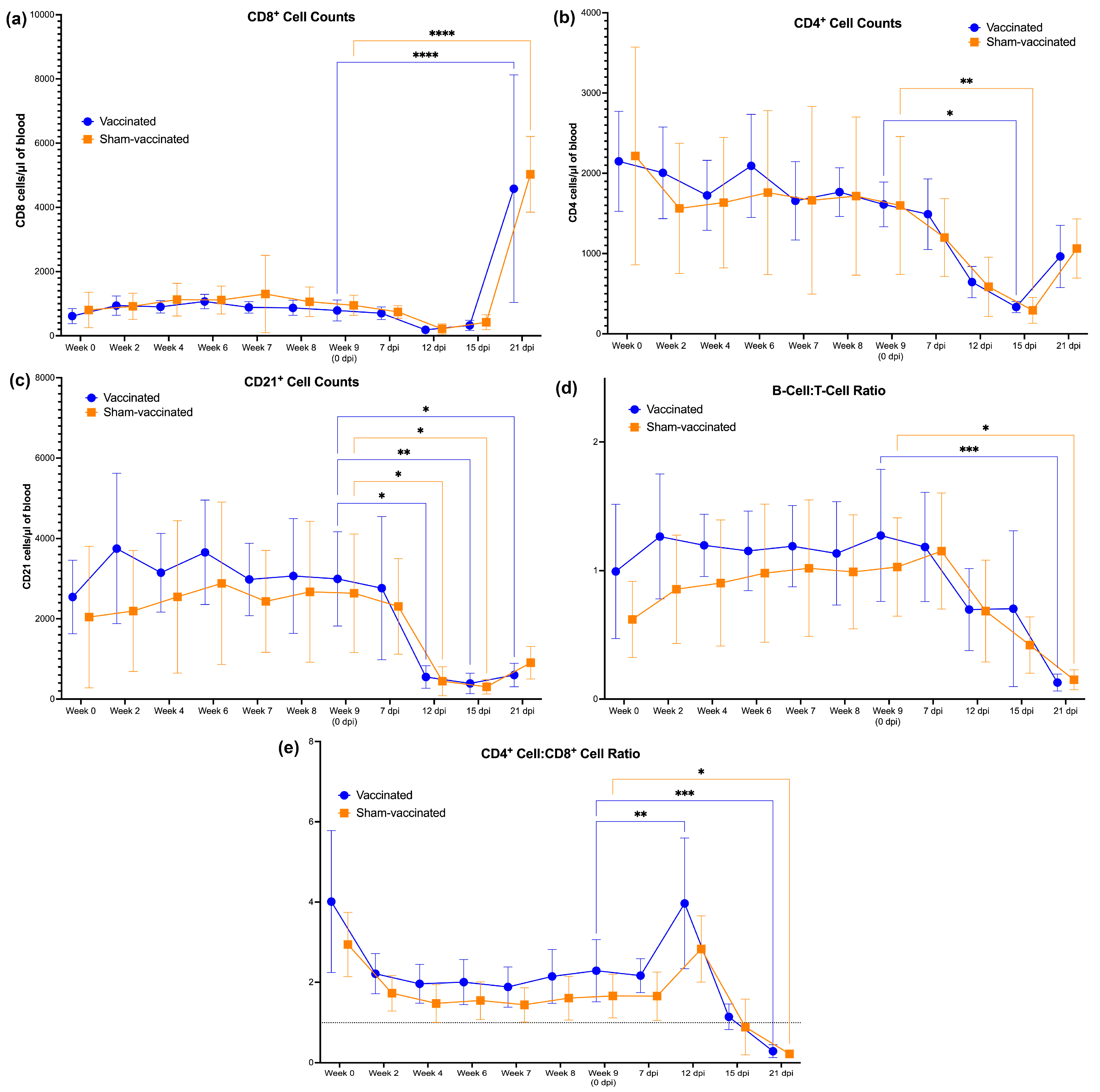
3.3. Changes in the CD4+ and CD21+ Cells following Vaccination
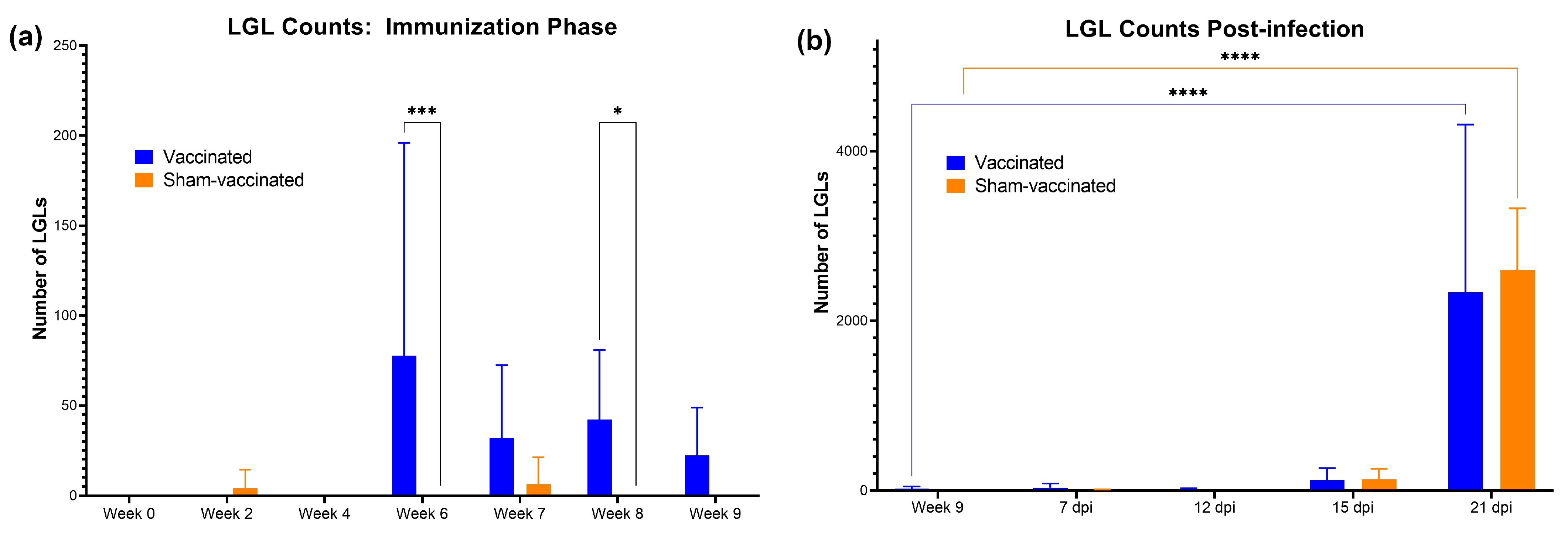
3.4. Vaccination and C. felis Infection Increased the LGL Levels in Cats
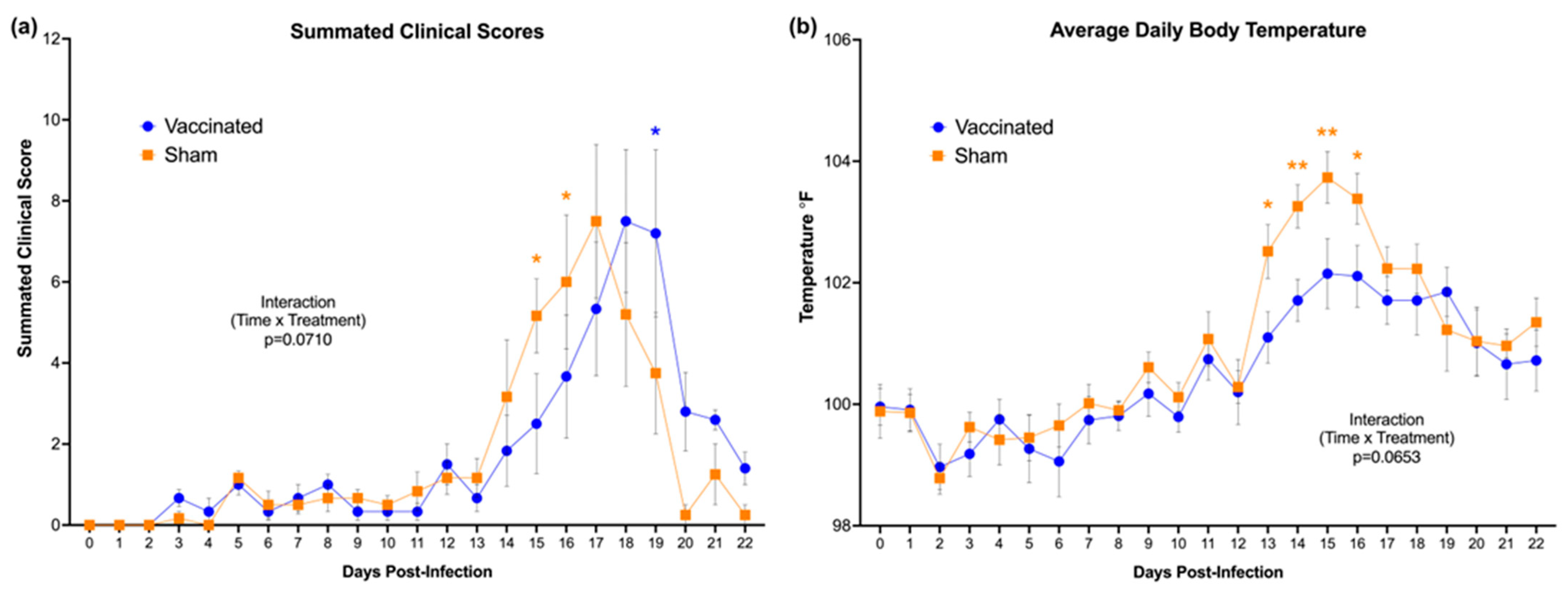
3.5. Delayed Onset of Cytauxzoonosis and Decreased Febrility
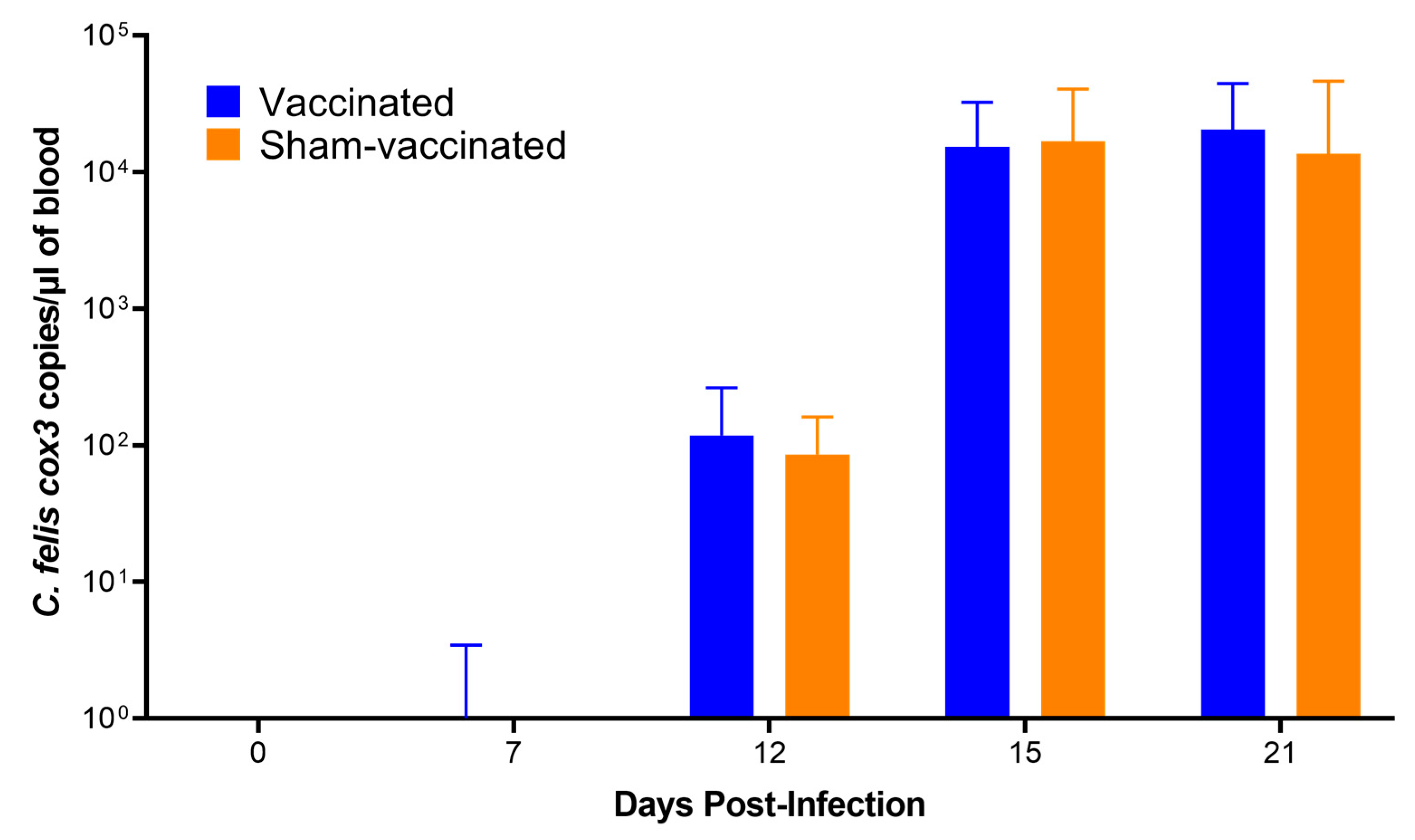
3.6. Outcome of C. felis Challenge
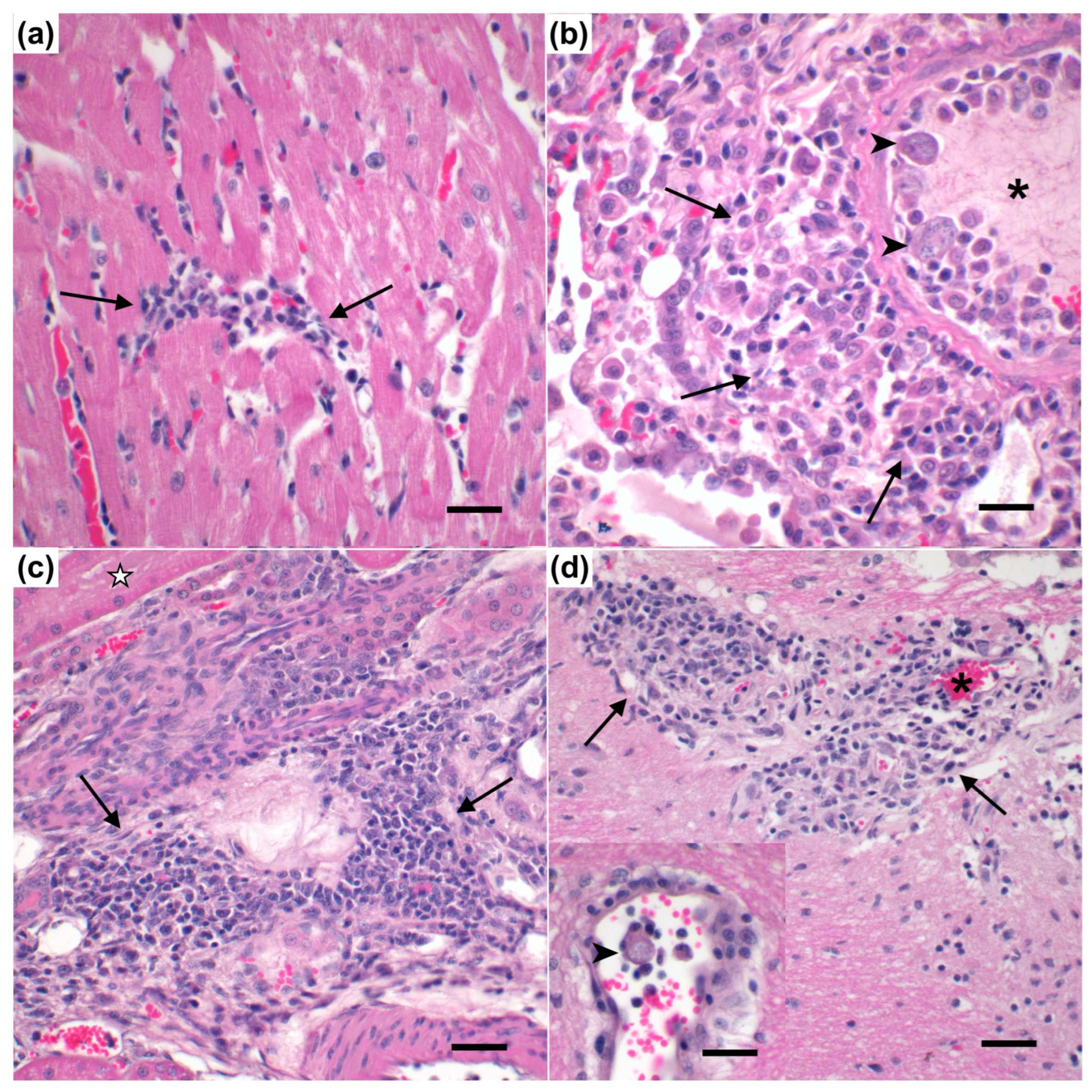
3.7. Pathology of C. felis Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donoghue, P. Haemoprotozoa: Making biological sense of molecular phylogenies. Int. J. Parasitol. Parasites Wildl. 2017, 6, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Sanders, T.L.; Weerarathne, P.; Meinkoth, J.H.; Miller, C.A.; Scimeca, R.C.; Almazán, C. Cytauxzoonosis in North America. Pathogens 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M.-J.; Aldasoro, E.; Calvo-Cano, A.; Picado, A.; Muñoz, J.; Gascon, J. Blood and Tissue Protozoa. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1751–1762. [Google Scholar]
- Raimundo, J.M.; Guimarães, A.; André, M.R.; Baldani, C.D. Cytauxzoon felis DNA detection in healthy cats from Rio de Janeiro, Brazil. J. Parasitol. 2021, 107, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Panait, L.C.; Stock, G.; Globokar, M.; Balzer, J.; Groth, B.; Mihalca, A.D.; Pantchev, N. The first case of feline cytauxzoonosis in Germany: Clinical description and molecular confirmation. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Antognoni, M.T.; Rocconi, F.; Ravagnan, S.; Vascellari, M.; Capelli, G.; Miglio, A.; Di Tommaso, M. Cytauxzoon sp. Infection and Coinfections in Three Domestic Cats in Central Italy. Vet. Sci. 2022, 9, 50. [Google Scholar] [CrossRef]
- Willi, B.; Meli, M.L.; Cafarelli, C.; Gilli, U.O.; Kipar, A.; Hubbuch, A.; Riond, B.; Howard, J.; Schaarschmidt, D.; Regli, W.; et al. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: A tale that started more than two decades ago. Parasites Vectors 2022, 15, 19. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Villaescusa, A.; Ayllón, T.; Rodríguez-Franco, F.; Baneth, G.; Calleja-Bueno, L.; García-Sancho, M.; Agulla, B.; Sainz, Á. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasites Vectors 2017, 10, 112. [Google Scholar] [CrossRef]
- Leclaire, S.; Menard, S.; Berry, A. Molecular characterization of Babesia and Cytauxzoon species in wild South-African meerkats. Parasitology 2015, 142, 543–548. [Google Scholar] [CrossRef]
- Naidenko, S.V.; Erofeeva, M.N.; Sorokin, P.A.; Gershov, S.O.; Yakovenko, N.P.; Botvinovskaya, A.S.; Alekseeva, G.S. The First Case of Cytauxzoon spp. in Russia: The Parasite Conquers Eurasia. Animals 2022, 12, 593. [Google Scholar] [CrossRef]
- Malangmei, L.; Ajith Kumar, K.G.; Nandini, A.; Bora, C.A.F.; Varghese, A.; Amrutha, B.M.; Kurbet, P.S.; Pradeep, R.K.; Nimisha, M.; Deepa, C.K.; et al. Molecular Characterization of Hemoparasites and Hemoplasmas Infecting Domestic Cats of Southern India. Front. Vet. Sci. 2021, 7, 597598. [Google Scholar] [CrossRef]
- Wagner, J.E. A fatal cytauxzoonosis-like disease in cats. J. Am. Vet. Med. Assoc. 1976, 168, 585–588. [Google Scholar] [PubMed]
- Wikander, Y.M.; Reif, K.E. Cytauxzoon felis: An Overview. Pathogens 2023, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Tarigo, J.L.; Scholl, E.H.; Bird, D.M.; Brown, C.C.; Cohn, L.A.; Dean, G.A.; Levy, M.G.; Doolan, D.L.; Trieu, A.; Nordone, S.K.; et al. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS ONE 2013, 8, e71233. [Google Scholar] [CrossRef]
- Sherrill, M.K.; Cohn, L.A. Cytauxzoonosis: Diagnosis and treatment of an emerging disease. J. Feline Med. Surg. 2015, 17, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.A.; Birkenheuer, A.J.; Brunker, J.D.; Ratcliff, E.R.; Craig, A.W. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J. Vet. Intern. Med. 2011, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Thomas, J.E.; Arther, R.G.; Hostetler, J.A.; Raetzel, K.L.; Meinkoth, J.H.; Little, S.E. Efficacy of an imidacloprid 10%/flumethrin 4.5% collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol. Res. 2013, 112, 11–20. [Google Scholar] [CrossRef]
- Reichard, M.V.; Rugg, J.J.; Thomas, J.E.; Allen, K.E.; Barrett, A.W.; Murray, J.K.; Herrin, B.H.; Beam, R.A.; King, V.L.; Vatta, A.F. Efficacy of a topical formulation of selamectin plus sarolaner against induced infestations of Amblyomma americanum on cats and prevention of Cytauxzoon felis transmission. Vet. Parasitol. 2019, 270, S31–S37. [Google Scholar] [CrossRef]
- Nene, V.; Iams, K.P.; Gobright, E.; Musoke, A.J. Characterisation of the gene encoding a candidate vaccine antigen of Theileria parva sporozoites. Mol. Biochem. Parasitol. 1992, 51, 17–27. [Google Scholar] [CrossRef]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.L.; Sherrill, M.K.; Outi, H.K.; Scholl, E.H.; Bird, D.M.; Vigil, A.; Hung, C.; Nakajima, R.; et al. Identification of Cytauxzoon felis antigens via protein microarray and assessment of expression library immunization against cytauxzoonosis. Clin. Proteom. 2018, 15, 44. [Google Scholar] [CrossRef]
- Nemerow, G.; Flint, J. Lessons learned from adenovirus (1970–2019). FEBS Lett. 2019, 593, 3395–3418. [Google Scholar] [CrossRef]
- Crampton, A.; Vanniasinkam, T. Parasite vaccines: The new generation. Infect. Genet. Evol. 2007, 7, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Mendes, É.A.; Fonseca, F.G.; Casério, B.M.; Colina, J.P.; Gazzinelli, R.T.; Caetano, B.C. Recombinant vaccines against T. gondii: Comparison between homologous and heterologous vaccination protocols using two viral vectors expressing SAG1. PLoS ONE 2013, 8, e63201. [Google Scholar] [CrossRef] [PubMed]
- Halbroth, B.R.; Sebastian, S.; Salman, A.M.; Ulaszewska, M.; Gola, A.; Longley, R.J.; Janse, C.J.; Khan, S.M.; Hill, A.V.; Spencer, A.J. Preclinical development and assessment of viral vectors expressing a fusion antigen of Plasmodium falciparum LSA1 and LSAP2 for efficacy against liver-stage malaria. Infect. Immun. 2020, 88, e00573-19. [Google Scholar] [CrossRef]
- Svitek, N.; Saya, R.; Awino, E.; Munyao, S.; Muriuki, R.; Njoroge, T.; Pellé, R.; Ndiwa, N.; Poole, J.; Gilbert, S.; et al. An Ad/MVA vectored Theileria parva antigen induces schizont-specific CD8+ central memory T cells and confers partial protection against a lethal challenge. NPJ Vaccines 2018, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.M.J.; Molinari, M.P.; Gravisaco, M.J.; Paoletta, M.S.; Montenegro, V.N.; Wilkowsky, S.E. Evaluation of different heterologous prime–boost immunization strategies against Babesia bovis using viral vectored and protein-adjuvant vaccines based on a chimeric multi-antigen. Vaccine 2016, 34, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.M.W.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Roy-Chowdhury, J.; Horwitz, M.S. Evolution of adenoviruses as gene therapy vectors. Mol. Ther. 2002, 5, 340–344. [Google Scholar] [CrossRef]
- Kozarsky, K.F.; Wilson, J.M. Gene therapy: Adenovirus vectors. Curr. Opin. Genet. Dev. 1993, 3, 499–503. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2021, 42, 100432. [Google Scholar] [CrossRef]
- Provine, N.M.; Amini, A.; Garner, L.C.; Spencer, A.J.; Dold, C.; Hutchings, C.; Silva Reyes, L.; FitzPatrick, M.E.; Chinnakannan, S.; Oguti, B.; et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Vupputuri, S.; Tayebi, L.; Hikkaduwa Koralege, R.S.; Nigatu, A.; Mozafari, M.; Mishra, A.; Liu, L.; Ramsey, J.D. Polyethylene glycol–modified DOTAP: Cholesterol/adenovirus hybrid vectors have improved transduction efficiency and reduced immunogenicity. J. Nanoparticle Res. 2021, 23, 37. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Yoshida, K.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Alam, A.; Emran, T.B.; Amelia, F.; et al. Adeno-associated virus as an effective malaria booster vaccine following adenovirus priming. Front. Immunol. 2019, 10, 730. [Google Scholar] [CrossRef]
- Mebatsion, T.; Linz, P.R.; Moore, J. Recombinant Adenoviral Feline Leukemia Virus Vaccine and Use Thereof. Patent WO2010004043A1, 14 January 2010. [Google Scholar]
- Kao, Y.F.; Peake, B.; Madden, R.; Cowan, S.R.; Scimeca, R.C.; Thomas, J.E.; Reichard, M.V.; Ramachandran, A.; Miller, C.A. A probe-based droplet digital polymerase chain reaction assay for early detection of feline acute cytauxzoonosis. Vet. Parasitol. 2021, 293, 109428. [Google Scholar] [CrossRef]
- Reichard, M.V.; Meinkoth, J.H.; Edwards, A.C.; Snider, T.A.; Kocan, K.M.; Blouin, E.F.; Little, S.E. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 2009, 161, 110–115. [Google Scholar] [CrossRef]
- Reichard, M.V.; Edwards, A.C.; Meinkoth, J.H.; Snider, T.A.; Meinkoth, K.R.; Heinz, R.E.; Little, S.E. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J. Med. Entomol. 2010, 47, 890–896. [Google Scholar] [CrossRef]
- Rudd, J.M.; Tamil Selvan, M.; Cowan, S.; Kao, Y.F.; Midkiff, C.C.; Narayanan, S.; Ramachandran, A.; Ritchey, J.W.; Miller, C.A. Clinical and histopathologic features of a feline SARS-CoV-2 infection model are analogous to acute COVID-19 in humans. Viruses 2021, 13, 1550. [Google Scholar] [CrossRef]
- Tamil Selvan, M.; Gunasekara, S.; Xiao, P.; Griffin, K.; Cowan, S.R.; Narayanan, S.; Ramachandran, A.; Hagen, D.E.; Ritchey, J.W.; Rudd, J.M.; et al. SARS CoV-2 (Delta variant) infection kinetics and immunopathogenesis in domestic cats. Viruses 2022, 14, 1207. [Google Scholar] [CrossRef]
- Kao, Y.F.; Spainhour, R.; Cowan, S.R.; Nafe, L.; Birkenheuer, A.; Reichard, M.V.; Miller, C.A. A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis. Pathogens 2022, 11, 1183. [Google Scholar] [CrossRef]
- Swart, A.; Maas, M.; de Vries, A.; Cuperus, T.; Opsteegh, M. Bayesian Binary Mixture Models as a Flexible Alternative to Cut-Off Analysis of ELISA Results, a Case Study of Seoul Orthohantavirus. Viruses 2021, 13, 1155. [Google Scholar] [CrossRef]
- Meyer, E.L.; Jenkins, C.; Rengarajan, K. NIH Guidelines April 2019. Appl. Biosaf. 2019, 24, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Bielefeldt-Ohmann, H.; MacMillan, M.; Huitron-Resendiz, S.; Henriksen, S.; Elder, J.; VandeWoude, S. Strain-specific viral distribution and neuropathology of feline immunodeficiency virus. Vet. Immunol. Immunopathol. 2011, 143, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Boegler, K.; Carver, S.; MacMillan, M.; Bielefeldt-Ohmann, H.; VandeWoude, S. Pathogenesis of oral FIV infection. PLoS ONE 2017, 12, e0185138. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.; Vogels, R.; Custers, J.H.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef]
- Gonin, P.; Fournier, A.; Oualikene, W.; Moraillon, A.; Eloit, M. Immunization trial of cats with a replication-defective adenovirus type 5 expressing the ENV gene of feline immunodeficiency virus. Vet. Microbiol. 1995, 45, 393–401. [Google Scholar] [CrossRef]
- Tarigo, J. The Cytauxzoon felis Genome: A Guide to Vaccine Candidate Antigen Discovery for Cytauxzoonosis. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2013. [Google Scholar]
- Khana, D.B.; Peterson, D.S.; Stanton, J.B.; Schreeg, M.E.; Birkenheuer, A.J.; Tarigo, J.L. Genetic conservation of Cytauxzoon felis antigens and mRNA expression in the schizont life-stage. Vet. Parasitol. 2018, 263, 49–53. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Sacks, D.L. Why does immunity to parasites take so long to develop? Nat. Rev. Immunol. 2010, 10, 80–81. [Google Scholar] [CrossRef]
- Stafford, J.L.; Neumann, N.F.; Belosevic, M. Macrophage-mediated innate host defense against protozoan parasites. Crit. Rev. Microbiol. 2002, 28, 187–248. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Goncalves, R.; Christensen, S.M.; Mosser, D.M. Humoral immunity in leishmaniasis–Prevention or promotion of parasite growth? Cytokine X 2020, 2, 100046. [Google Scholar] [CrossRef] [PubMed]
- Villarino, N.; Schmidt, N.W. CD8+ T cell responses to plasmodium and intracellular parasites. Curr. Immunol. Rev. 2013, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Dotiwala, F.; Mulik, S.; Polidoro, R.B.; Ansara, J.A.; Burleigh, B.A.; Walch, M.; Gazzinelli, R.T.; Lieberman, J. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat. Med. 2016, 22, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.O.; Carvalho, L.P.; Graff, J.W.; Beiting, D.P.; Ruthel, G.; Roos, D.S.; Betts, M.R.; Goldschmidt, M.H.; Wilson, M.E.; de Oliveira, C.I.; et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013, 9, e1003504. [Google Scholar] [CrossRef]
- Cohn, L.A. Cytauxzoonosis. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1211–1224. [Google Scholar] [CrossRef]
- Lloret, A.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Lutz, H.; et al. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 637–641. [Google Scholar] [CrossRef]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’–a review. J. Infect. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef]
- Zambello, R.; Semenzato, G. Large granular lymphocyte disorders: New etiopathogenetic clues as a rationale for innovative therapeutic approaches. Haematologica 2009, 94, 1341–1345. [Google Scholar] [CrossRef]
- Loughran, T.J. Clonal diseases of large granular lymphocytes. Blood 1993, 82, 1–14. [Google Scholar] [CrossRef]
- Fattizzo, B.; Bellani, V.; Pasquale, R.; Giannotta, J.A.; Barcellini, W. Large Granular Lymphocyte Expansion in Myeloid Diseases and Bone Marrow Failure Syndromes: Whoever Seeks Finds. Front. Oncol. 2021, 11, 748610. [Google Scholar] [CrossRef]
- Sprague, W.S.; Apetrei, C.; Avery, A.C.; Peskind, R.L.; Vandewoude, S. Large granular lymphocytes are universally increased in human, macaque, and feline lentiviral infection. Vet. Immunol. Immunopathol. 2015, 167, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Snead, E.C. Large granular intestinal lymphosarcoma and leukemia in a dog. Can. Vet. J. 2007, 48, 848–851. [Google Scholar] [PubMed]
- Gurunathan, S.; Prussin, C.; Sacks, D.L.; Seder, R.A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 1998, 4, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.F.; Gomes, V.O.; dos Santos Maciel, M.O.; Melo, L.M.; Venturin, G.L.; Bragato, J.P.; Rebech, G.T.; de Oliveira Santos, C.; Nascimento de Oliveira, B.M.; Gileno de Sá Oliveira, G.; et al. Combined in vitro IL-12 and IL-15 stimulation promotes cellular immune response in dogs with visceral leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008021. [Google Scholar] [CrossRef]
- Wilairatana, P.; Kwankaew, P.; Kotepui, K.U.; Kotepui, M. Low interleukin-12 levels concerning severe malaria: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 9345. [Google Scholar] [CrossRef]
- Thomas, J.E.; Staubus, L.; Goolsby, J.L.; Reichard, M.V. Ectoparasites of free-roaming domestic cats in the central United States. Vet. Parasitol. 2016, 228, 17–22. [Google Scholar] [CrossRef]
- Saleh, M.E.; Sundstrom, K.D.; Duncan, K.T.; Ientile, M.M.; Jordy, J.; Ghosh, P.; Little, S.E. Show us your ticks: A survey of ticks infesting dogs and cats across the USA. Parasites Vectors 2019, 12, 595. [Google Scholar] [CrossRef]

| Clinical Parameter | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Rectal Temperature | 100.5 to 102.5 °F | 102.5 to 103.5 °F | 103.6 to 105 °F | >105.1 °F |
| MM CRT | Pink, moist <2 s | Pale pink, tacky 2–3 s | Pale, tacky to dry 2–3 s | Pale or bright red >3 s |
| Activity | Normal | Mild reduction when disturbed * (mild lethargy) | Moderate reduction when disturbed * (moderate lethargy) | Little to no activity Disturbed * and reduced activity stimulated ** |
| Appetite | Normal | Reduced interest in food; 1 d not eating | Markedly reduced interest in food; 2 d not eating | Anorexia; 3 d not eating |
| Respiratory Effort | Normal resting respiratory rate and normal effort | Mild tachypnea (>35 breaths per min); no overt increase in effort otherwise | Moderate tachypnea (>40 breaths per min); moderate increase in effort | Marked tachypnea (>45 breaths per min); marked effort or dyspnea |
| Icterus | None | Mild scleral icterus; none in skin or MM | Subtle icterus in sclera, skin, and MM | Obvious icterus in sclera, skin, and MM |
| Dehydration | Euhydrated | ~5%—Mild, semi-dry oral mucus membranes, normal eye | ~6 to 8%—moderate degree of decreased skin turgor; dry oral mucous membranes | >8%—marked degree of decreased skin turgor, dry mucous membranes, weak and rapid pulse, slow CRT, notable mental depression |
| Pain | ≤2.0 | 2.0 to 2.25 | 2.5 to 2.75 | ≥3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerarathne, P.; Maker, R.; Huang, C.; Taylor, B.; Cowan, S.R.; Hyatt, J.; Tamil Selvan, M.; Shatnawi, S.; Thomas, J.E.; Meinkoth, J.H.; et al. A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats. Vaccines 2023, 11, 573. https://doi.org/10.3390/vaccines11030573
Weerarathne P, Maker R, Huang C, Taylor B, Cowan SR, Hyatt J, Tamil Selvan M, Shatnawi S, Thomas JE, Meinkoth JH, et al. A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats. Vaccines. 2023; 11(3):573. https://doi.org/10.3390/vaccines11030573
Chicago/Turabian StyleWeerarathne, Pabasara, Rebekah Maker, Chaoqun Huang, Brianne Taylor, Shannon R. Cowan, Julia Hyatt, Miruthula Tamil Selvan, Shoroq Shatnawi, Jennifer E. Thomas, James H. Meinkoth, and et al. 2023. "A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats" Vaccines 11, no. 3: 573. https://doi.org/10.3390/vaccines11030573
APA StyleWeerarathne, P., Maker, R., Huang, C., Taylor, B., Cowan, S. R., Hyatt, J., Tamil Selvan, M., Shatnawi, S., Thomas, J. E., Meinkoth, J. H., Scimeca, R., Birkenheuer, A., Liu, L., Reichard, M. V., & Miller, C. A. (2023). A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats. Vaccines, 11(3), 573. https://doi.org/10.3390/vaccines11030573







