Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Analysis
2.2. Statistical Analysis
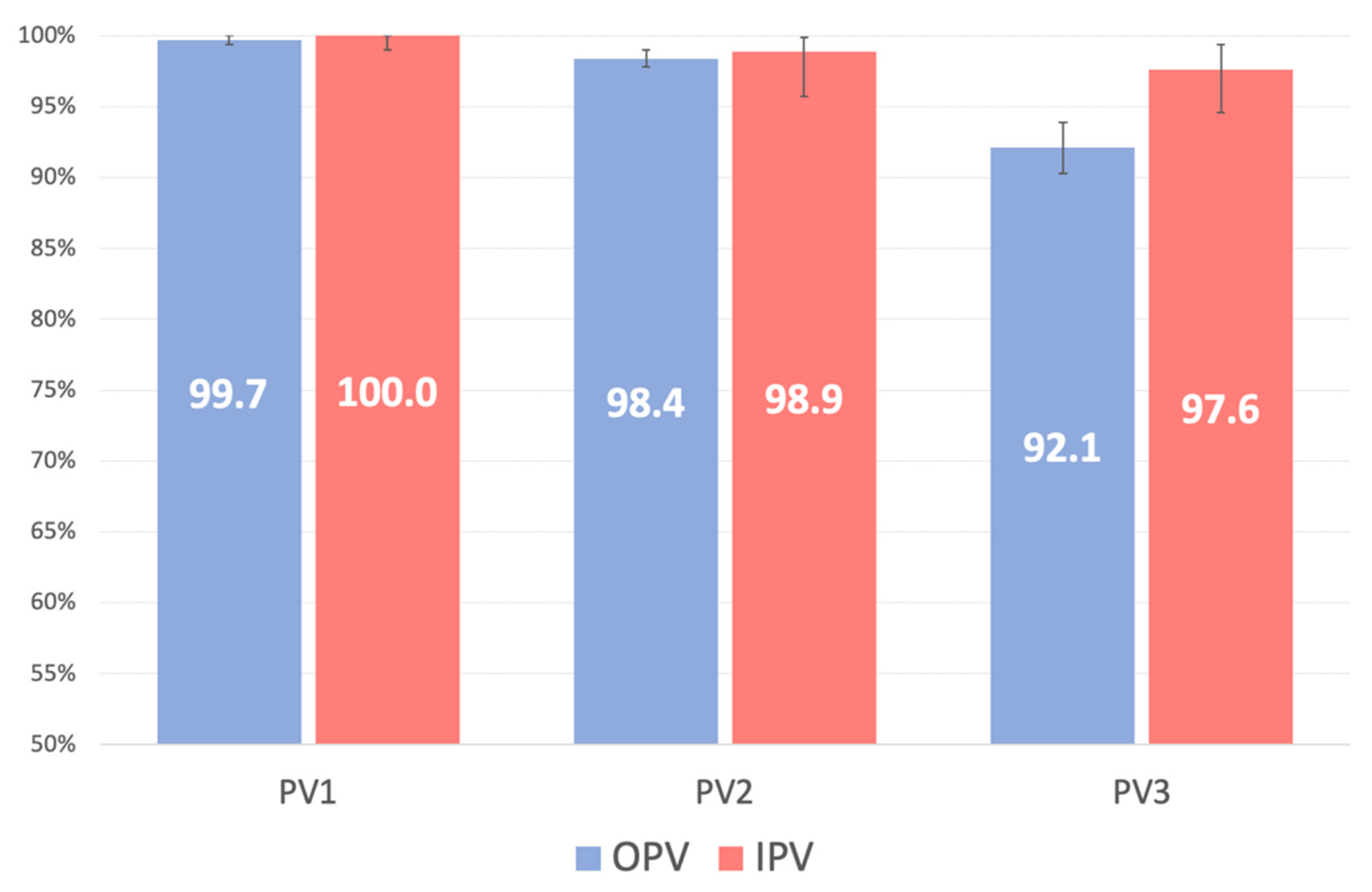
3. Results
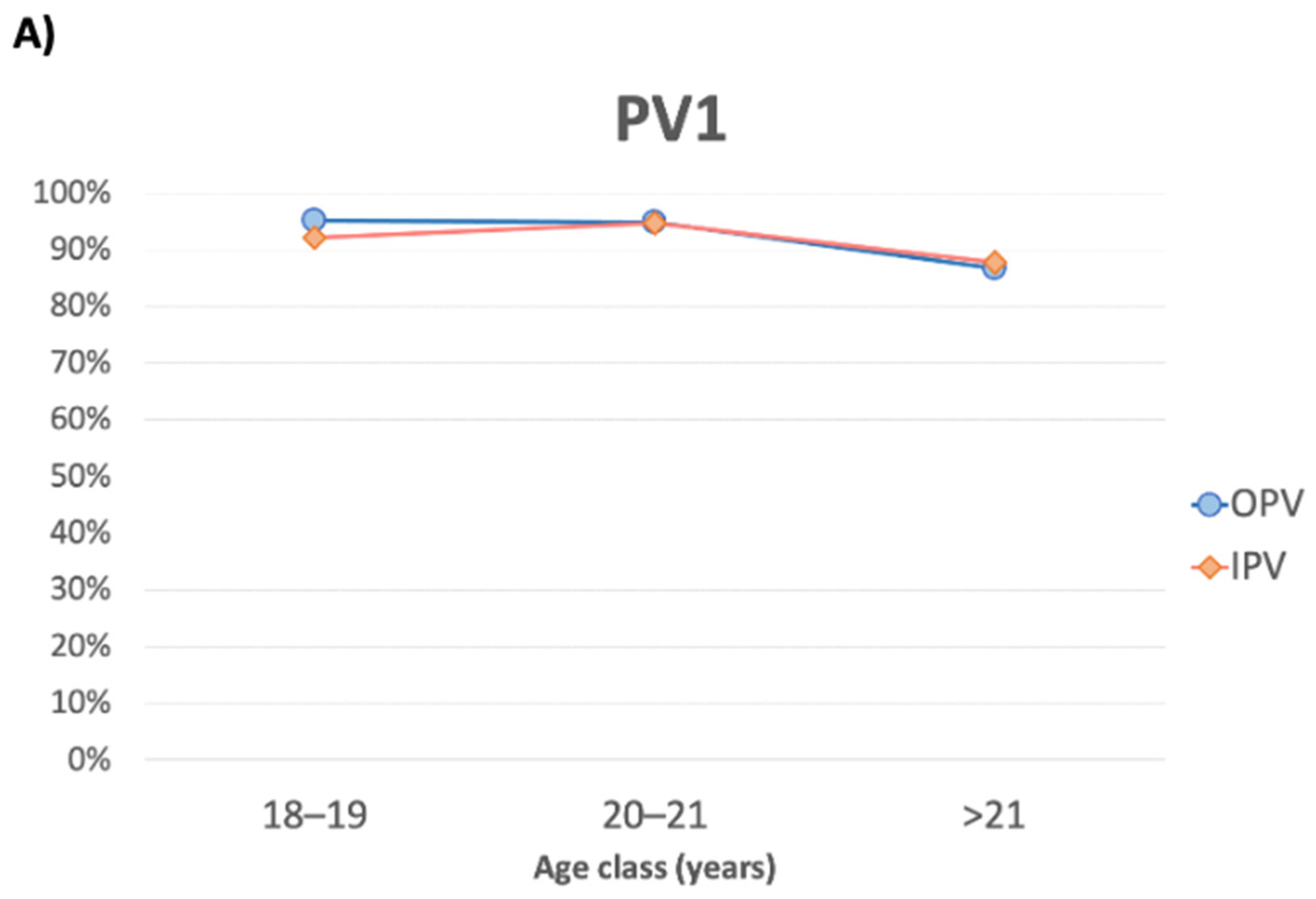
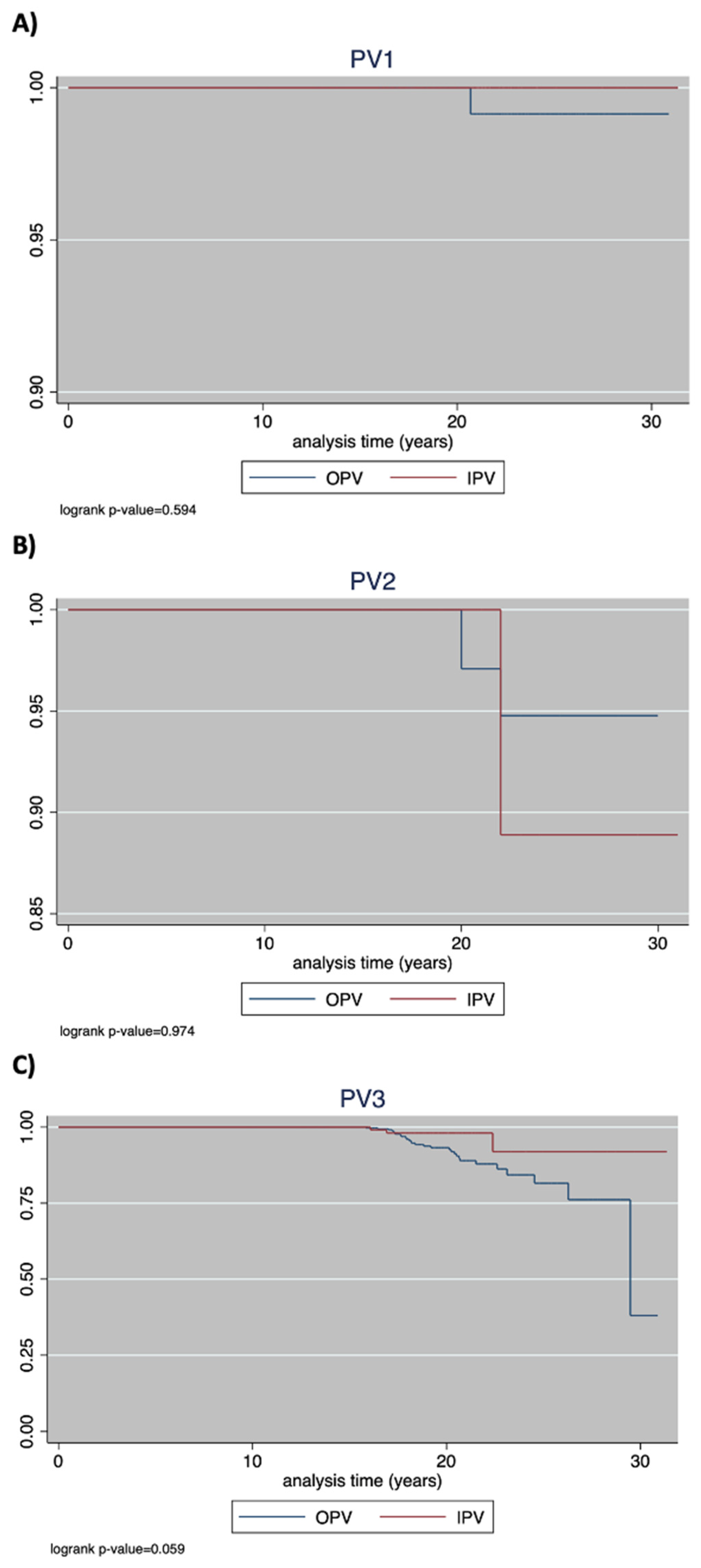
3.1. PV1
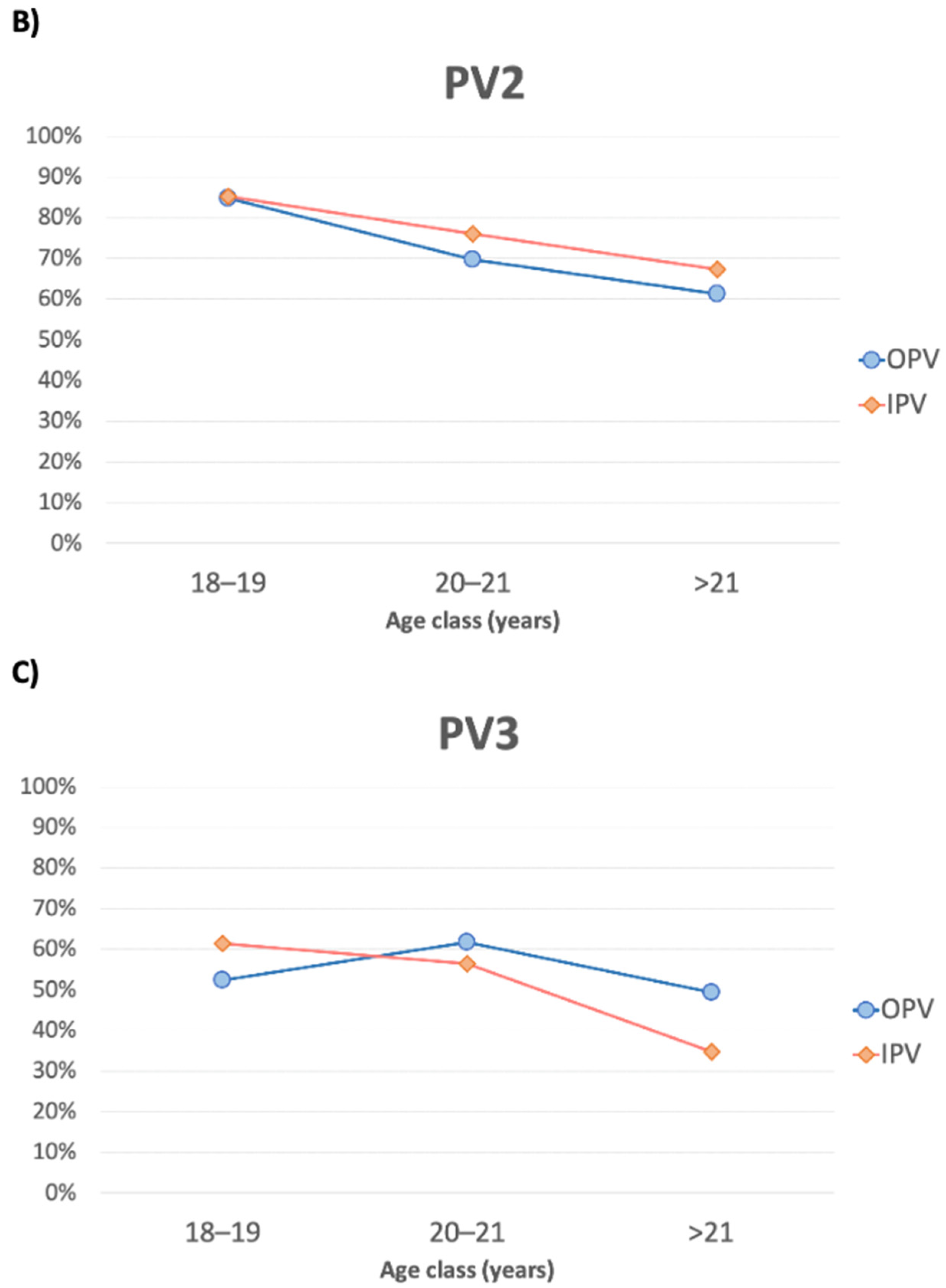
3.2. PV2
3.3. PV3
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Poliomyelitis. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 14 January 2021).
- Bandyopadhyay, A.S.; Gast, C.; Rivera, L.; Sáez-Llorens, X.; Oberste, M.S.; Weldon, W.C.; Modlin, J.; Clemens, R.; Clemens, S.A.C.; Jimeno, J.; et al. Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: A randomised open-label, multicentre, phase 3, non-inferiority trial. Lancet Infect. Dis. 2020, 21, 559–568. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Wild Poliovirus List. List of Wild Poliovirus by Country and Year. Available online: http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list (accessed on 15 January 2021).
- CDC. Who We Are: CDC and the Global Polio Eradication Initiative. Available online: https://www.cdc.gov/polio/who/index.htm (accessed on 18 January 2021).
- Quinn V, J.M.; Dhabalia, T.J.; Roslycky, L.L.; Wilson, V.J.M.; Hansen, J.C.; Hulchiy, O.; Golubovskaya, O.; Buriachyk, M.; Vadim, K.; Zauralskyy, R.; et al. COVID-19 at War: The Joint Forces Operation in Ukraine. Disaster Med. Public Health Prep. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crovari, P. Public Health History Corner History of polio vaccination in Italy. Ital. J. Public Health 2010, 7, 3. [Google Scholar]
- Italian Ministry of Health. Decree Law 7 June 2017, n. 73, Urgent Provisions on Vaccination Prevention, as Amended by the Conversion Law July 31, 2017. Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=60201 (accessed on 3 January 2021).
- WHO European Region. Certification of the Region’s Polio-Free Status in 2002. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/poliomyelitis/activities/certification-and-maintenance-of-polio-free-status-in-the-european-region/european-regional-commission-for-the-certification-of-poliomyelitis-eradication/certification-of-the-regions-polio-free-status-in-2002 (accessed on 21 January 2021).
- WHO. Persistence of Protective Antibodies following Immunization with OPV and IPV. Available online: https://www.who.int/immunization/polio_grad_duration_protection.pdf (accessed on 27 October 2020).
- CDC. Vaccines and Preventable Diseases. Polio Vaccine Effectiveness and Duration of Protection. Available online: https://www.cdc.gov/vaccines/vpd/polio/hcp/effectiveness-duration-protection.html (accessed on 29 January 2021).
- CDC. Pink book. Epidemiology and Prevention of Vaccine-Preventable Diseases. Poliomyelitis. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/polio.html#vaccines (accessed on 5 January 2021).
- WHO. Expanded Programme on Immunization (WHO-EPI-CDC-Polio-90.1). In Manual for the Virological Investigation of Poliomyelitis; WHO: Geneva, Switzerland, 1990; pp. 44–65. [Google Scholar]
- Tafuri, S.; Prato, R.; Martinelli, D.; Calvario, A.; Bozzi, A.; Labianca, M.; Patti, A.; Lopalco, P.L.; Germinario, C. Serological survey on immunity status against polioviruses in children and adolescents living in a border region, Apulia (Southern Italy). BMC Infect. Dis. 2008, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, Y.; Deng, X.; Yue, C.; Tang, X.; Li, Y.; Liu, Y.; Yin, Z.; Zhang, G.; Chen, Z.; et al. Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: An open-label, randomised, controlled trial. Lancet Infect. Dis. 2020, 20, 1071–1079. [Google Scholar] [CrossRef]
- Mahamud, A.; Kamadjeu, R.; Webeck, J.; Mbaeyi, C.; Baranyikwa, M.T.; Birungi, J.; Nurbile, Y.; Ehrhardt, D.; Shukla, H.; Chatterjee, A.; et al. Effectiveness of oral polio vaccination against paralytic poliomyelitis: A matched case-control study in Somalia. J. Infect. Dis. 2014, 210 (Suppl. 1), S187–S193. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Jadhav, M.; John, T.J. Efficacy of inactivated poliovirus vaccine in India. Bull. World Health Organ. 1983, 61, 689–692. [Google Scholar] [PubMed]
- Bianchi, F.P.; De Nitto, S.; Stefanizzi, P.; Larocca, A.M.V.; Germinario, C.A.; Tafuri, S. Long time persistence of antibodies against Mumps in fully MMR immunized young adults: An Italian retrospective cohort study. Hum. Vaccin. Immunother. 2020, 16, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; Vimercati, L.; Mansi, F.; De Nitto, S.; Stefanizzi, P.; Rizzo, L.A.; Fragnelli, G.R.; Cannone, E.S.S.; De Maria, L.; Larocca, A.M.V.; et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: The experience of a university hospital in southern Italy. Am. J. Infect. Control. 2020, 48, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; De Nitto, S.; Stefanizzi, P.; Larocca, A.M.V.; Germinario, C.A.; Tafuri, S. Immunity to rubella: An Italian retrospective cohort study. BMC Public Health 2019, 19, 1490. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-term immunogenicity after measles vaccine vs. wild infection: An Italian retrospective cohort study. Hum. Vaccin. Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.; Stefanati, A.; Baldovin, T.; Roman, A.; Baldo, V.; Gabutti, G. Assessment of seroprevalence against poliovirus among Italian adolescents and adults. Hum. Vaccin. Immunother. 2019, 15, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, L.; Affanni, P.; Verrotti di Pianella, C.; Colucci, M.E.; Tanzi, M.L. Immunity status against poliomyelitis in childbearing women in a province of northern Italy. A cross-sectional analysis. Ann. Ig. 2013, 25, 427–433. [Google Scholar] [PubMed]
- Baldo, V.; Baldovin, T.; Cocchio, S.; Lazzari, R.; Saracino, E.; Bertoncello, C.; Buja, A.; Trevisan, A. Seroepidemiology of polioviruses among university students in northern Italy. Clin. Vaccine Immunol. 2012, 19, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Chironna, M.; Martinelli, D.; Sallustio, A.; Prato, R.; Germinario, C. Surveillance of poliovirus circulation among refugees in Italy, 2008–2011. J. Travel Med. 2012, 19, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Germinario, C.; Gallone, M.S.; Tafuri, S. Migrant health: The Apulian model. Epidemiol. Prev. 2015, 39 (Suppl. 1), 76–80. [Google Scholar] [PubMed]
- Lopalco, P.L. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol. Infect. 2017, 145, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Acute Flaccid Paralysis Surveillance. Available online: http://www.salute.gov.it/portale/malattieInfettive/dettaglioContenutiMalattieInfettive.jsp?lingua=italiano&id=820&area=Malattie%20infettive&menu=sorveglianza#:~:text=Il%20sistema%20di%20sorveglianza%20delle%20Paralisi%20Flaccide%20acute%20(PFA)%2C,mostrano%20sintomatologia%20identica%20alla%20polio (accessed on 21 January 2021).
- Italian Ministry of Health. National Plan of Vaccinal Prevention (PNPV) 2017–2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 23 January 2021).
| Variable | PV1 | PV2 | PV3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPV | IPV | Total | p-Value | OPV | IPV | Total | p-Value | OPV | IPV | Total | p-Value | |
| Susceptible; n (%; 95%CI) | 1 (0.27; 0.00–1.50) | 0 (0.00; 0.00–2.95) | 1 (0.20; 0.01–1.12) | 1.000 | 4 (1.59; 0.43–4.01) | 1 (1.08; 0.03–5.85) | 5 (1.45; 0.47–3.35) | 1.000 | 29 (7.85; 5.33–11.09) | 3 (2.44; 0.51–6.96) | 32 (6.50; 4.49–9.06) | 0.022 |
| Protective titer; n (%) | 0.859 | 0.179 | 0.328 | |||||||||
| low | 31/368 (8.4) | 11/123 (8.9) | 42/491 (8.6) | 72/248 (29.0) | 20/92 (21.7) | 92/340 (27.1) | 144/340 (42.4) | 57/120 (47.5) | 201/460 (43.7) | |||
| high | 337/368 (91.6) | 112/123 (91.1) | 449/491 (91.4) | 176/248 (71.0) | 72/92 (78.3) | 248/340 (72.9) | 196/340 (57.6) | 63/120 (52.5) | 259/460 (56.3) | |||
| Determinant | aOR | 95%CI | p-Value |
|---|---|---|---|
| Group (IPV vs. OPV) | 3.34 | 1.00–11.20 | 0.050 |
| Sex (male vs. female) | 0.97 | 0.44–2.12 | 0.934 |
| Age (years) | 1.00 | 0.89–1.13 | 0.979 |
| Immune-related chronic disease (YES/NO) | 1.83 | 0.80–4.18 | 0.152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larocca, A.M.V.; Bianchi, F.P.; Bozzi, A.; Tafuri, S.; Stefanizzi, P.; Germinario, C.A. Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study. Vaccines 2022, 10, 1329. https://doi.org/10.3390/vaccines10081329
Larocca AMV, Bianchi FP, Bozzi A, Tafuri S, Stefanizzi P, Germinario CA. Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study. Vaccines. 2022; 10(8):1329. https://doi.org/10.3390/vaccines10081329
Chicago/Turabian StyleLarocca, Angela Maria Vittoria, Francesco Paolo Bianchi, Anna Bozzi, Silvio Tafuri, Pasquale Stefanizzi, and Cinzia Annatea Germinario. 2022. "Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study" Vaccines 10, no. 8: 1329. https://doi.org/10.3390/vaccines10081329
APA StyleLarocca, A. M. V., Bianchi, F. P., Bozzi, A., Tafuri, S., Stefanizzi, P., & Germinario, C. A. (2022). Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study. Vaccines, 10(8), 1329. https://doi.org/10.3390/vaccines10081329







