Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice
Abstract
:1. Introduction
2. Materials and Methods
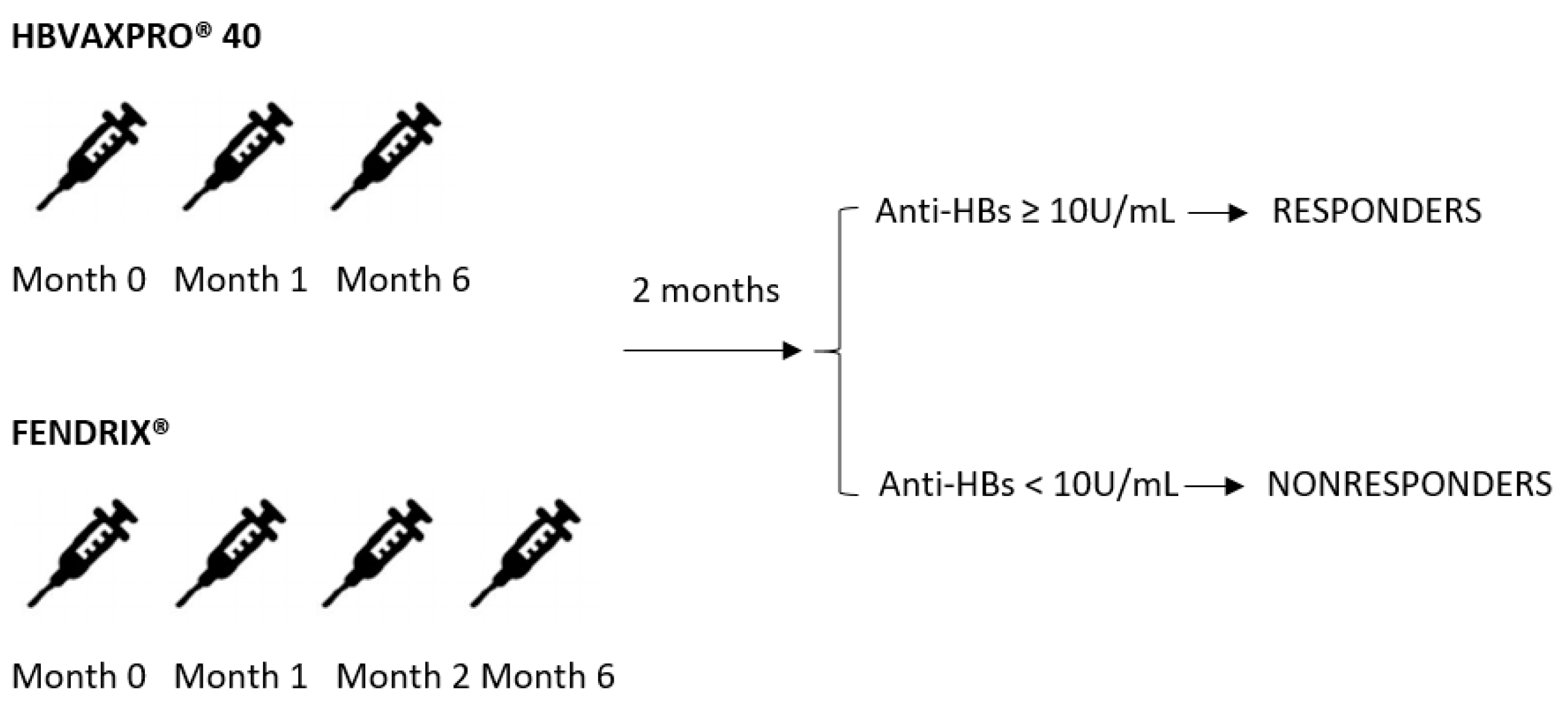
2.1. Study Overview
2.2. Participants and Eligibility Criteria
2.3. Intervention
2.4. Definitions
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
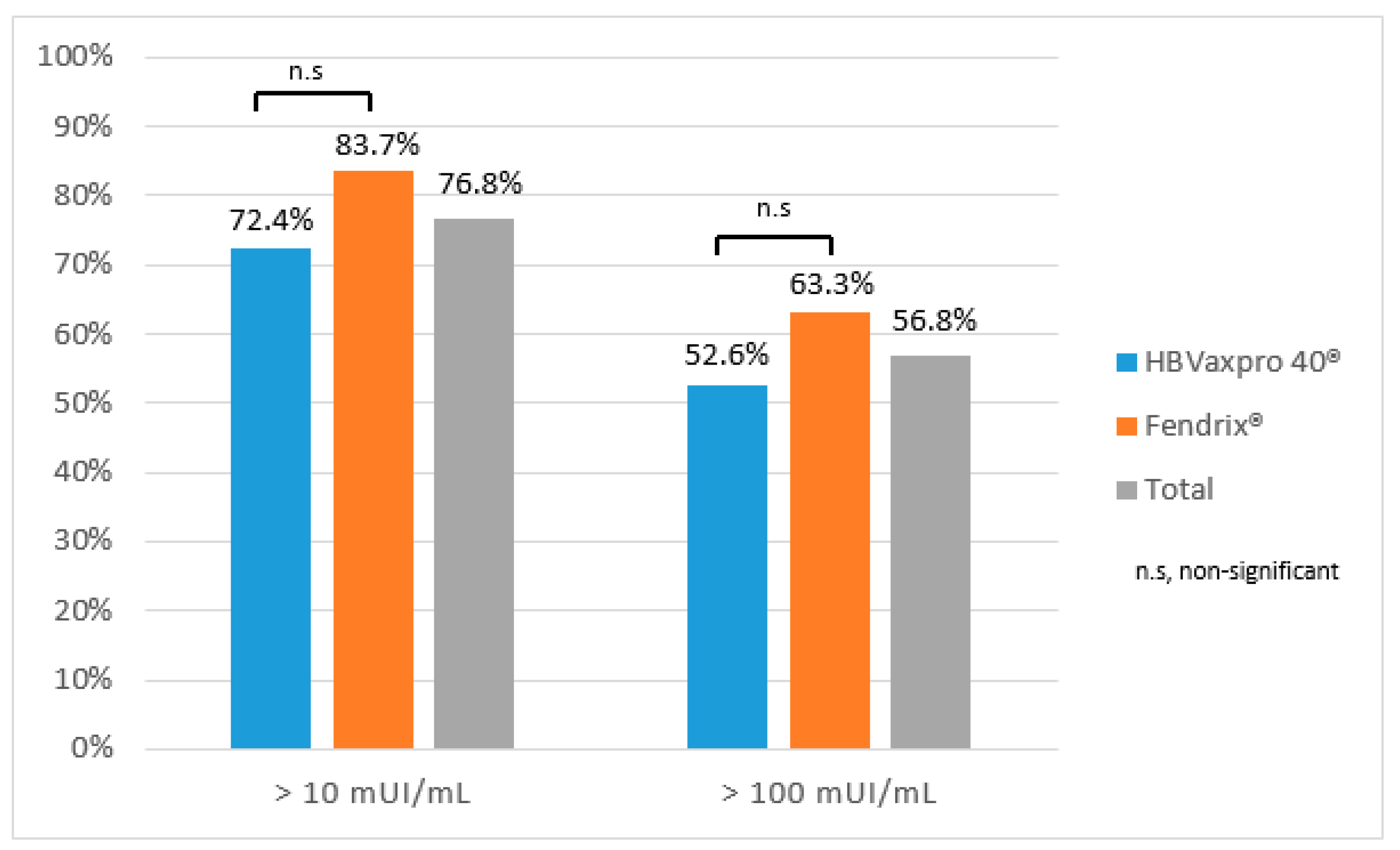
3.2. Vaccination Efficacy
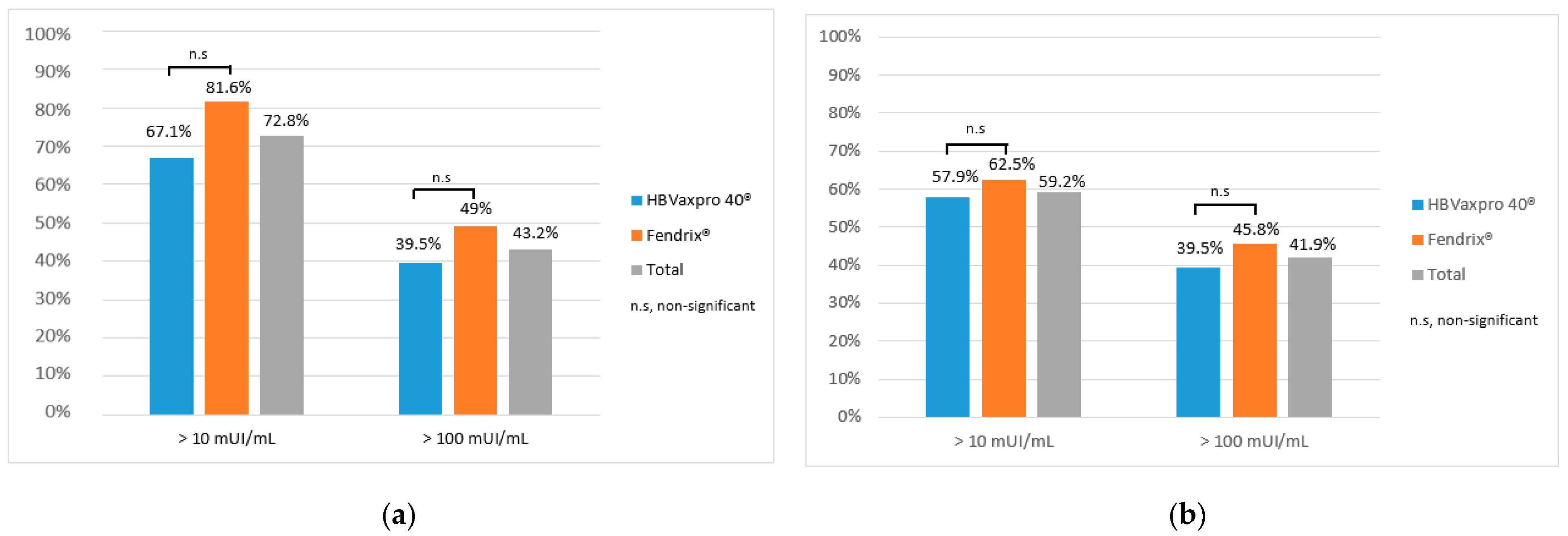
3.3. Vaccination Efficacy at Six and Twelve Months
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonnel, A.R.; Bunchorntavakul, C.; Reddy, K.R. Immune Dysfunction and Infections in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Varasa, T.D.A.; Ruano, J.J.S.; Vela, A.G.; Rodríguez, R.G.; Gutiérrez, M.R.; Perez, G.D.L.C.; Moreno, A.Z.G.; Jiménez, J.M.C. Eficacia y seguridad de la vacunación para la hepatitis A y la hepatitis B en pacientes con hepatopatía crónica. Gastroenterol. Hepatol. 2009, 32, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.R.; Hachem, C.Y.; Kanwal, F.; Mei, M.; El-Serag, H.B. Meeting vaccination quality measures for hepatitis A and B virus in patients with chronic hepatitis C infection. Hepatology 2010, 53, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wörns, M.A.; Teufel, A.; Kanzler, S.; Shrestha, A.; Victor, A.; Otto, G.; Lohse, A.W.; Galle, P.R.; Höhler, T. Incidence of HAV and HBV Infections and Vaccination Rates in Patients With Autoimmune Liver Diseases. Am. J. Gastroenterol. 2008, 103, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.J. Vaccinating patients with chronic liver disease. Gastroenterol. Hepatol. 2012, 8, 120–122. [Google Scholar]
- Poland, G.A.; Jacobson, R.M. Prevention of Hepatitis B with the Hepatitis B Vaccine. N. Engl. J. Med. 2004, 351, 2832–2838. [Google Scholar] [CrossRef]
- Poorolajal, J.; Hooshmand, E. Booster dose vaccination for preventing hepatitis B. Cochrane Database Syst. Rev. 2016, 2016, CD008256. [Google Scholar] [CrossRef]
- World Health Organization Hepatitis B vaccines: WHO position paper, July 2017—Recommendations. Vaccine 2019, 37, 223–225. [CrossRef] [PubMed]
- Engler, S.H.; Sauer, P.W.; Golling, M.; Klar, E.A.; Benz, C.; Stremmel, W.; Kallinowski, B. Immunogenicity of two accelerated hepatitis B vaccination protocols in liver transplant candidates. Eur. J. Gastroenterol. Hepatol. 2001, 13, 363–367. [Google Scholar] [CrossRef] [PubMed]
- De Maria, N.; Idilman, R.; Colantoni, A.; Van Thiel, D.H. Increased effective immunogenicity to high-dose and short-interval hepatitis B virus vaccination in individuals with chronic hepatitis without cirrhosis. J. Viral Hepat. 2001, 8, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Castells, L.; Esteban, R. Hepatitis B vaccination in liver transplant candidates. Eur. J. Gastroenterol. Hepatol. 2001, 13, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Idilman, R.; Colantoni, A.; De Maria, N.; Ustun, C.; Sam, R.; Ing, T.S.; Akan, H.; Koc, H.; Van Thiel, D.H. Impaired antibody response rates after high dose short interval hepatitis B virus vaccination of immunosuppressed individuals. Hepatogastroenterology 2003, 50, 217–221. [Google Scholar] [PubMed]
- Fonseca, M.O.; Pang, L.W.; Cavalheiro, N.D.P.; Barone, A.A.; Lopes, M.H. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005, 23, 2902–2908. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Menchén, L.; García-Sánchez, V.; Marín, I.; Villagrasa, J.R.; Chaparro, M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 35, 1379–1385. [Google Scholar] [CrossRef]
- Carey, W.; Pimentel, R.; Westveer, M.K.; Vogt, D.; Broughan, T. Failure of hepatitis B immunization in liver transplant recipients: Results of a prospective trial. Am. J. Gastroenterol. 1990, 85, 1590–1592. [Google Scholar]
- Van Thiel, D.H.; El-Ashmawy, L.; Love, K.; Gavaler, J.S.; Starzl, T.E. Response to hepatitis B vaccination by liver transplant candidates. Am. J. Dig. Dis. 1992, 37, 1245–1249. [Google Scholar] [CrossRef]
- Horlander, J.C.; Boyle, N.; Manam, R.; Schenk, M.; Herring, S.; Kwo, P.Y.; Lumeng, L.; Chalasani, N. Vaccination against Hepatitis B in Patients with Chronic Liver Disease Awaiting Liver Transplantation. Am. J. Med Sci. 1999, 318, 304–307. [Google Scholar] [CrossRef]
- Idilman, R.; Maria, N.; Colantoni, A.; Nadir, A.; Thiel, D.H.; De, M.N. The effect of high dose and short interval HBV vaccination in individuals with chronic hepatitis C. Am. J. Gastroenterol. 2002, 97, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tajes, S.; Pocurull, A.; Lens, S.; Mariño, Z.; Olivas, I.; Soy, G.; Alonso, A.; Vilella, A.; Forns, X. Efficacy of an accelerated double-dose hepatitis B vaccine regimen in patients with cirrhosis. J. Viral Hepat. 2021, 28, 1019–1024. [Google Scholar] [CrossRef]
- Nevens, F.; Zuckerman, J.N.; Burroughs, A.K.; Jung, M.-C.; Bayas, J.M.; Kallinowski, B.; Rivas, E.F.; Duvoux, C.; Neuhaus, P.; Saliba, F.; et al. Immunogenicity and safety of an experimental adjuvanted hepatitis B candidate vaccine in liver transplant patients. Liver Transplant. 2006, 12, 1489–1495. [Google Scholar] [CrossRef]
- Bonazzi, P.R.; Bacchella, T.; Freitas, A.C.; Osaki, K.T.; Lopes, M.H.; Freire, M.P.; Machado, M.C.; Abdala, E. Double-dose hepatitis B vaccination in cirrhotic patients on a liver transplant waiting list. Braz. J. Infect. Dis. 2008, 12, 306–309. [Google Scholar] [CrossRef]
- Arslan, M.; Wiesner, R.H.; Sievers, C.; Egan, K.; Zein, N.N. Double-dose accelerated hepatitis B vaccine in patients with end-stage liver disease. Liver Transplant. 2001, 7, 314–320. [Google Scholar] [CrossRef]
- Aziz, A.; Aziz, S.; Li, D.S.; Murphy, L.; Leone, N.; Kennedy, M.; Dhillon, S.; Van Thiel, D.H. Efficacy of repeated high-dose hepatitis B vaccine (80 mug) in patients with chronic liver disease. J. Viral Hepat. 2005, 13, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Domingo, I.G.; Acevedo, J.P.; Vargas, A.A.; Cuadra, A.R.; Ríos, M.F.; Martin, J.S.; Mota, M.S.; Gallego, A.G.; Artacho, G.S. Response to Vaccination Against Hepatitis B Virus with a Schedule of Four 40-μg Doses in Cirrhotic Patients Evaluated for Liver Transplantation: Factors Associated with a Response. Transplant. Proc. 2012, 44, 1499–1501. [Google Scholar] [CrossRef]
- Daryani, N.E.; Nassiri-Toosi, M.; Rashidi, A.; Khodarahmi, I. Immunogenicity of recombinant hepatitis B virus vaccine in patients with and without chronic hepatitis C virus infection: A case-control study. World J. Gastroenterol. 2007, 13, 294–298. [Google Scholar] [CrossRef]
- Arbizu, E.A.; Marugán, R.B.; Grijalba, J.Y.; Serrano, P.L.; Gil Grande, L.; Terrón, S.D.C. Intramuscular versus intradermal administration of anti-hepatitis B vaccine in non-cirrhotic hepatitis C patients. Vaccine 2003, 21, 2747–2750. [Google Scholar] [CrossRef]
- Wiedmann, M.; Liebert, U.G.; Oesen, U.; Porst, H.; Wiese, M.; Schroeder, S.; Halm, U.; Mössner, J.; Berr, F. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology 2000, 31, 230–234. [Google Scholar] [CrossRef]
- Yang, S.; Tian, G.; Cui, Y.; Ding, C.; Deng, M.; Yu, C.; Xu, K.; Ren, J.; Yao, J.; Li, Y.; et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 2016, 6, 27251. [Google Scholar] [CrossRef]
- Barr, T.; Helms, C.; Grant, K.; Messaoudi, I. Opposing effects of alcohol on the immune system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 65, 242–251. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Reiss, G.; Keeffe, E.B. Hepatitis vaccination in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2004, 19, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Villagrasa, J.R.; Rodríguez-Nogueiras, A.; Chaparro, M. Kinetics of Anti–Hepatitis B Surface Antigen Titers After Hepatitis B Vaccination in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.K.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Cardell, K.; Åkerlind, B.; Sällberg, M.; Frydén, A. Excellent Response Rate to a Double Dose of the Combined Hepatitis A and B Vaccine in Previous Nonresponders to Hepatitis B Vaccine. J. Infect. Dis. 2008, 198, 299–304. [Google Scholar] [CrossRef]

| Total n = 125 | Hbvaxpro® 40 n = 76 | Fendrix ® n = 49 | p | |
|---|---|---|---|---|
| Age (Mean ± SD) | 61.8 ± 9.4 | 62.2 ± 10.0 | 61.2 ± 8.5 | n.s |
| Gender (male) (n, %) | 72 (57.6) | 42 (55.3) | 30 (61.2) | n.s |
| Smokers (n, %) | 85 (68) | 62 (81.6) | 23 (46.9) | 0.001 |
| Active alcohol intake (n, %) | 40 (32) | 20 (26.3) | 20 (40.8) | n.s |
| Diabetes (n, %) | 27 (21.6) | 18 (23.7) | 9 (18.4) | n.s |
| BMI (Mean ± SD) | 29.5 ± 5.5 | 30.2 ± 5.3 | 28.3 ± 5.7 | n.s |
| Overweight (BMI ≥ 25) (n, %) | 105 (84) | 69 (90.7) | 36 (73.5) | n.s |
| Chronic liver disease | n.s | |||
| Etiology (n, %) | ||||
| Alcohol | 44 (35.2) | 25 (32.9) | 19 (38.8) | |
| MAFLD | 36 (28.8) | 19 (25) | 17 (34.7) | |
| HCV | 25 (20) | 18 (23.7) | 7 (14.3) | |
| AIH/PBC | 20 (16) | 14 (18.4) | 6 (12.2) | |
| Liver cirrhosis (n, %) | 54 (43.2) | 28 (36.8) | 26 (53.1) | n.s |
| Child–Pugh (n, %) | n.s | |||
| A | 41 (75.9) | 22 (78.6) | 19 (73.1) | |
| B | 13 (24.1) | 6 (21.4) | 7 (26.9) | |
| Stage by elastography | n.s | |||
| (n, %) | ||||
| F0–F2 | 70 (56) | 48 (64) | 22 (45.8) | |
| F3–F4 | 52 (42.4) | 27 (36) | 26 (54.2) | |
| Index for Liver | n.s | |||
| Fibrosis (Mean ± SD) | ||||
| FIB-4 | 2.5 ± 2.1 | 2.3 ± 1.8 | 2.9 ± 2.7 | |
| APRI | 0.7 ± 0.8 | 0.6 ± 0.5 | 0.8 ± 1.0 | |
| FORNS | 6.4 ± 2.2 | 6.2 ± 2.1 | 6.7 ± 2.2 | |
| US signs of portal hypertension (n, %) | 30 (24) | 14 (18.4) | 16 (32.7) | n.s |
| Immunosuppressive treatment (n, %) | 11 (8.8) | 7 (9.2) | 4 (8.2) | n.s |
| Responders n = 96 | Nonresponders n = 29 | OR (95% CI) | p | |
|---|---|---|---|---|
| Age (years) (Mean ± SD) | 61.3 ± 9.9 | 63.4 ± 7.5 | - | n.s |
| Gender (male) (n, %) | 51 (53.1) | 21 (72.4) | 1.36 (1.01–1.82) | n.s |
| Smoker (n, %) | 64 (66.7) | 21 (72.4) | 1.08 (0.83–1.41) | n.s |
| Active alcohol intake (n, %) | 26 (27.1) | 20 (26.3) | 1.78 (1.08–2.93) | 0.04 |
| Diabetes (n, %) | 17(17.7) | 10 (34.5) | 1.94 (1.00–3.77) | n.s |
| BMI (Mean ± SD) | 29.3 ± 5.6 | 30.1 ± 5.1 | - | n.s |
| Overweight (BMI ≥ 25) (n, %) | 79 (82.3) | 26 (89.7) | 1.08 (0.93–1.27) | n.s |
| Chronic liver disease | ||||
| Etiology (n, %) | ||||
| Alcohol | 26 (27.1) | 18 (62.1) | 2.29 (1.48–3.53) | 0.001 |
| MAFLD | 29 (30.2) | 7 (24.1) | 0.79 (0.39–1.63) | n.s |
| HCV | 23 (24) | 2 (6.9) | 0.28 (0.07–1.14) | n.s |
| AIH/PBC | 18 (18.8) | 2 (6.9) | 0.36 (0.09–1.49) | n.s |
| Liver cirrhosis (n, %) | 30 (31.3) | 24 (82.8) | 2.64 (1.88–3.72) | <0.001 |
| Child–Pugh (n, %) | ||||
| A | 23(76.7) | 18 (75) | 0.97 (0.72–1.32) | n.s |
| B | 7 (23.3) | 6 (25) | 1.07 (0.41–2.76) | n.s |
| Stage by elastography | ||||
| (n, %) | ||||
| F0–F2 | 64 (67.4) | 6 (21.4) | 0.31 (0.15–0.65) | n.s |
| F3–F4 | 31 (32.6) | 22 (78.6) | 2.40 (1.70–3.41) | <0.001 |
| Index for Liver Fibrosis | - | |||
| (Mean ± SD) | ||||
| FIB-4 | 2.2 ± 2.1 | 3.8 ± 2.3 | n.s | |
| APRI | 0.6 ± 0.7 | 1.1 ± 0.7 | n.s | |
| FORNS | 6.1 ± 2.1 | 7.8 ± 1.9 | n.s | |
| US signs of portal hypertension (n, %) | 17 (17.7) | 13 (44.8) | 2.53 (1.40–4.57) | 0.005 |
| Responders n = 96 | Nonresponders n = 29 | OR (95% CI) | p | |
|---|---|---|---|---|
| Diabetes (n, %) | 15 (16.5) | 12 (35.3) | 2.14 (1.11–4.09) | 0.029 |
| Alcohol etiology (n, %) | 25 (27.5) | 19 (55.9) | 2.03 (1.30–3.18) | 0.022 |
| Liver cirrhosis (n, %) | 29 (31.9) | 25 (73.5) | 2.30 (1.61–3.31) | <0.001 |
| Stage F3–F4 by elastography (n, %) | 30 (33.3) | 23 (69.7) | 2.09 (1.44–3.02) | <0.001 |
| US signs of portal hypertension (n, %) | 15 (16.5) | 15 (44.1) | 2.67 (1.47–4.86) | 0.002 |
| Responders n = 96 | Nonresponders n = 29 | OR (95% CI) | p | |
|---|---|---|---|---|
| Alcohol etiology (n, %) | 18 (24.3) | 25 (51.0) | 2.09 (1.29–3.39) | 0.004 |
| Liver cirrhosis (n, %) | 19 (25.7) | 33 (67.3) | 2.67 (1.73–4.10) | <0.001 |
| Stage F3-F4 by elastography (n, %) | 20 (27.4) | 31 (64.6) | 2.40 (1.57–3.67) | <0.001 |
| US signs of portal hypertension (n, %) | 11 (14.9) | 18 (36.7) | 2.50 (1.30–4.80) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horta, D.; Forné, M.; Agustí, A.; Raga, A.; Martín-Cardona, A.; Hernández-Soto, J.M.; Ruiz-Ramírez, P.; Esteve-Comas, M. Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice. Vaccines 2022, 10, 1323. https://doi.org/10.3390/vaccines10081323
Horta D, Forné M, Agustí A, Raga A, Martín-Cardona A, Hernández-Soto JM, Ruiz-Ramírez P, Esteve-Comas M. Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice. Vaccines. 2022; 10(8):1323. https://doi.org/10.3390/vaccines10081323
Chicago/Turabian StyleHorta, Diana, Montserrat Forné, Anna Agustí, Agnes Raga, Albert Martín-Cardona, Juana María Hernández-Soto, Pablo Ruiz-Ramírez, and Maria Esteve-Comas. 2022. "Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice" Vaccines 10, no. 8: 1323. https://doi.org/10.3390/vaccines10081323
APA StyleHorta, D., Forné, M., Agustí, A., Raga, A., Martín-Cardona, A., Hernández-Soto, J. M., Ruiz-Ramírez, P., & Esteve-Comas, M. (2022). Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice. Vaccines, 10(8), 1323. https://doi.org/10.3390/vaccines10081323







