CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Animals, HSV-2, and Cell Lines
2.3. Prokaryotic Expression and Purification of gB
2.4. Mouse Immunization
2.5. Murine Sera, Vaginal Fluids, and Tissue Sampling
2.6. Ag-Specific Immunoglobulin (Ig) and Ig Isotyping ELISA
2.7. Virus Neutralization Assay
2.8. Ag-Specific Cytokine Assay
2.9. Immunohistochemistry of Colorectal Tissues
2.10. Chemotaxis Assay
2.11. Cell Surface Staining and Analysis of Murine Lymphocytes
2.12. Challenge, Scoring, and Virus Quantification
2.13. Statistical Analysis
3. Results
3.1. Codelivery of CCL28 with gB Plasmids Enhances gB-Specific, Th1-Polarized Humoral Responses
3.2. Codelivery of a Low Dose of CCL28 (LDCCL28) with gB Plasmids Promotes Enhanced Viral Neutralization Activities and Protective Effects Post Challenge
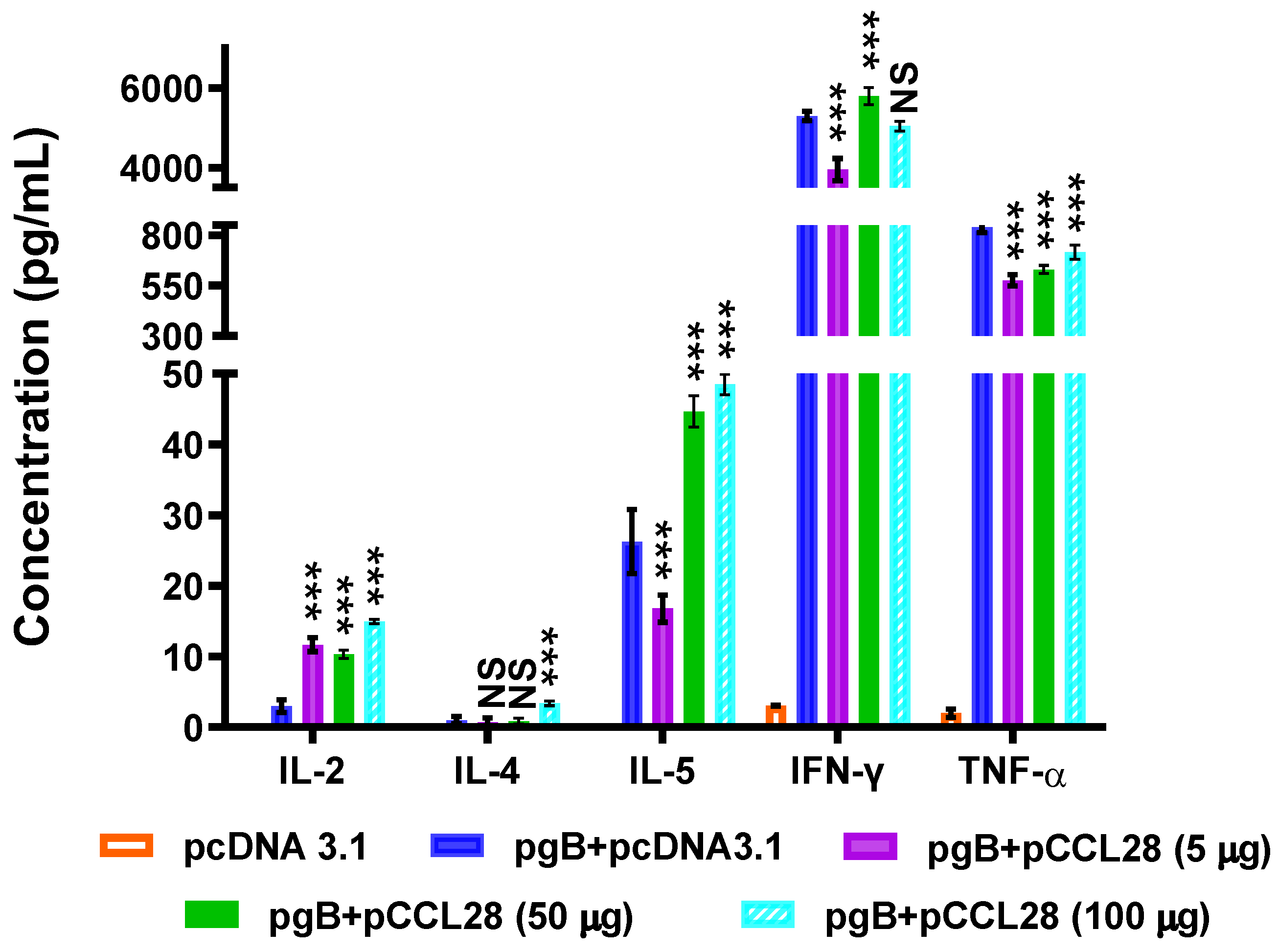
3.3. LDCCL28 Coimmunized with gB Plasmids Induce Ag-Specific Th1-Polarized Cellular Immune Responses
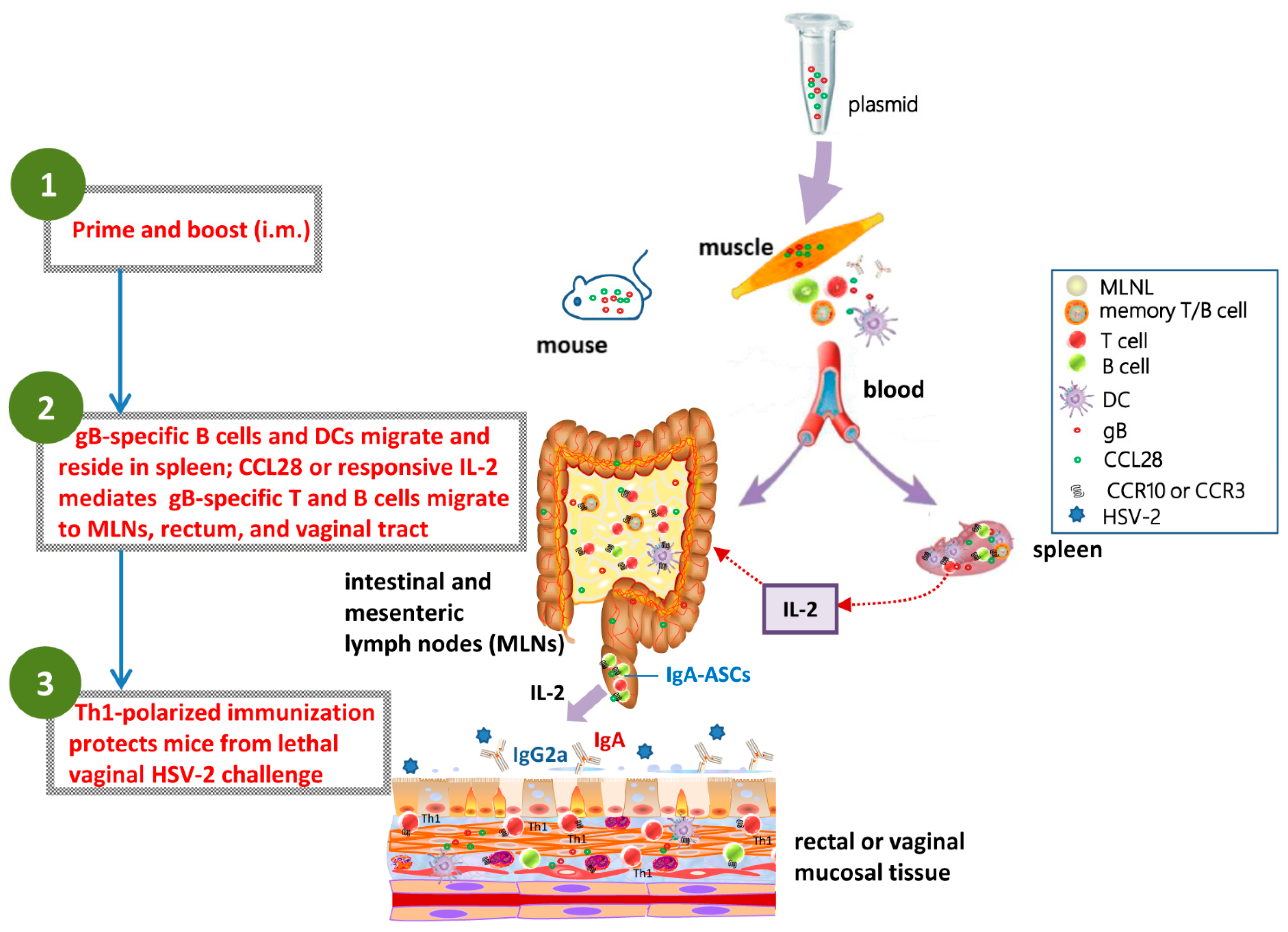
3.4. Coimmunization of LDCCL28 with gB Plasmids Enhances Immunocyte Migration and Settlement in Secondary Lymph Sites
3.5. Codelivery of LDCCL28 and gB Plasmids Enhances the Responsive Immunocytes Chemoattracting to Colorectal Mucosal and Secondary Lymphoid Sites
3.6. Codelivery of LDCCL28 and gB Plasmids Reduces Viral Shedding and Latency Post Lethal Vaginal Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Awasthi, S.; Friedman, H.M. An mRNA vaccine to prevent genital herpes. Transl. Res. 2022, 242, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Gottlieb, S.L. Biologic interactions between HSV-2 and HIV-1 and possible implications for HSV vaccine development. Vaccine 2019, 37, 7363–7371. [Google Scholar] [CrossRef]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Johnston, C.; Koelle, D.M.; Wald, A. HSV-2: In pursuit of a vaccine. J. Clin. Investig. 2011, 121, 4600–4609. [Google Scholar] [CrossRef] [Green Version]
- Kowalzik, F.; Schreiner, D.; Jensen, C.; Teschner, D.; Gehring, S.; Zepp, F. mRNA-based vaccines. Vaccines 2021, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Painful failure of promising genital herpes vaccine. Science 2010, 330, 304. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.P.; Muhammad, Z.S.; Wang, J.G.; Lin, W.; Guo, S.K.; Zhang, W. HSV-2 vaccine: Current status and insight into factors for developing an efficient vaccine. Viruses 2014, 6, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshak, J.O.; Dong, L.; Koelle, D.M. The murine intravaginal HSV-2 challenge model for investigation of DNA vaccines. Methods Mol. Biol. 2014, 1144, 305–327. [Google Scholar] [PubMed] [Green Version]
- Görander, S.; Harandi, A.M.; Lindqvist, M.; Bergström, T.; Liljeqvist, J.Å. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J. Virol. 2012, 86, 7544–7553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Hu, K.; Fu, M.; Deng, X.; Luo, S.; Tong, L.; Guan, X.; He, S.; Li, C.; Jin, W.; et al. CCL19 and CCL28 assist herpes simplex virus 2 glycoprotein D to induce protective systemic immunity against genital viral challenge. mSphere 2021, 6, e00058-21. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hu, K.; Deng, X.; Guan, X.; Luo, S.; Tong, L.; Du, T.; Fu, M.; Zhang, M.; Liu, Y.; et al. Immunization with HSV-2 gB-CCL19 fusion constructs protects mice against lethal vaginal challenge. J. Immunol. 2015, 195, 329–338. [Google Scholar] [CrossRef]
- Danilova, E.; Skrindo, I.; Gran, E.; Hales, B.J.; Smith, W.A.; Jahnsen, J.; Johansen, F.E.; Jahnsen, F.L.; Baekkevold, E.S. A role for CCL28-CCR3 in T-cell homing to the human upper airway mucosa. Mucosal Immunol. 2015, 8, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Deng, L.; Wang, B.-Z. CCL28 chemokine: An anchoring point bridging innate and adaptive immunity. Int. Immunopharmacol. 2017, 51, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hieshima, K.; Ohtani, H.; Shibano, M.; Izawa, D.; Nakayama, T.; Kawasaki, Y.; Shiba, F.; Shiota, M.; Katou, F.; Saito, T.; et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 2003, 170, 1452–1461. [Google Scholar] [CrossRef] [Green Version]
- Castelletti, E.; Lo Caputo, S.; Kuhn, L.; Borelli, M.; Gajardo, J.; Sinkala, M.; Trabattoni, D.; Kankasa, C.; Lauri, E.; Clivio, A.; et al. The mucosae-associated epithelial chemokine (MEC/CCL28) modulates immunity in HIV infection. PLoS ONE 2007, 2, e969. [Google Scholar] [CrossRef]
- Rainone, V.; Dubois, G.; Temchura, V.; Überla, K.; Clivio, A.; Nebuloni, M.; Lauri, E.; Trabattoni, D.; Veas, F.; Clerici, M. CCL28 induces mucosal homing of HIV-1-specific IgA-secreting plasma cells in mice immunized with HIV-1 virus-like particles. PLoS ONE 2011, 6, e26979. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Luo, S.; Tong, L.; Huang, X.; Jin, W.; Huang, W.; Du, T.; Yan, Y.; He, S.; Griffin, G.E.; et al. CCL19 and CCL28 augment mucosal and systemic immune responses to HIV-1 gp140 by mobilizing responsive immunocytes into secondary lymph nodes and mucosal tissue. J. Immunol. 2013, 191, 1935–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldon, Y.; Kratochvil, S.; Shattock, R.J.; McKay, P.F. Chemokine-adjuvanted plasmid DNA induces homing of antigen-specific and non-antigen-specific B and T cells to the intestinal and genital mucosae. J. Immunol. 2020, 204, 903–913. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Kraynyak, K.A.; Nagle, S.J.; Parkinson, R.M.; Zharikova, D.; Chattergoon, M.; Maguire, H.; Muthumani, K.; Ugen, K.; Weiner, D.B. Plasmids encoding the mucosal chemokines CCL27 and CCL28 are effective adjuvants in eliciting antigen-specific immunity in vivo. Gene. Ther. 2009, 17, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Virlogeux-Payant, I.; Chevaleyre, C.; Melo, S.; Zanello, G.; Salmon, H.; Meurens, F. CCL28 involvement in mucosal tissues protection as a chemokine and as an antibacterial peptide. Dev. Comp. Immunol. 2014, 44, 286–290. [Google Scholar] [CrossRef]
- He, J.; Thomas, M.A.; Anda, J.D.; Lee, M.W.; Why, E.V.; Simpson, P.; Wong, G.C.L.; Grayson, M.H.; Volkman, B.F.; Hupple, A.R. Chemokine CCL28 is a potent therapeutic agent for oropharyngeal candidiasis. Antimicrob. Agents Chemother. 2020, 64, e00210–e00220. [Google Scholar] [CrossRef]
- Toka, F.N.; Gierynska, M.; Rouse, B.T. Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J. Virol. 2003, 77, 12742–12752. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Dalai, S.K.; Wang, K.; Pesnicak, L.; Lau, T.Y.; Knipe, D.M.; Cohen, J.I.; Straus, S.E. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 2005, 79, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Clement, A.M.; Karen, L.E.; Michael, J.M.; Jerry, P.W. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 2002, 186, 1065–1073. [Google Scholar]
- Sin, J.I.; Kim, J.J.; Boyer, J.D.; Ciccarelli, R.B.; Higgins, T.J.; Weiner, D.B. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 1999, 73, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Jiang, D.; Zhang, L.; Yao, Z.; Chen, Z.; Yu, S.; Wang, X. Identification of B- and T-cell epitopes from glycoprotein B of herpes simplex virus 2 and evaluation of their immunogenicity and protection efficacy. Vaccine 2012, 30, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, L.M.; Schaerli, P.; Moser, B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 2005, 42, 799–809. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, K.L.; Jeong, J.B.; Shin, S.; Kim, S.H.; Kim, J.W. Expression of Chemokine CCL28 in Ulcerative Colitis Patients. Gut Liver 2021, 15, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Harasawa, H.; Yamada, Y.; Hieshima, K.; Jin, Z.; Nakayama, T.; Yoshie, O.; Shimizu, K.; Hasegawa, H.; Hayashi, T.; Imaizumi, Y.; et al. Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk. Lymphoma 2006, 47, 2163–2173. [Google Scholar] [CrossRef]
- Shao, D.D.; Meng, F.Z.; Liu, Y.; Xu, X.Q.; Wang, X.; Hu, W.H.; Hou, W.; Ho, W.Z. Poly(dA:dT) Suppresses HSV-2 Infection of Human Cervical Epithelial Cells Through RIG-I Activation. Front. Immunol. 2020, 11, 598884. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 2004, 25, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.; Burke, R.; Merigan, T. Liposome-formulated interleukin-2 as an adjuvant of recombinant HSV glycoprotein gD for the treatment of recurrent genital HSV-2 in guinea-pigs. Vaccine 1992, 10, 209–213. [Google Scholar] [CrossRef]
- Cheng, G.; Yu, A.; Malek, T.R. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 2011, 241, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.; Stewart, K.; Misirovs, R.; Lipworth, B. Targeting downstream type 2 cytokines or upstream epithelial alarmins for severe asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 1497–1505. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; Hu, Q. CCL19 and CCR7 expression, signaling pathways, and adjuvant functions in viral infection and prevention. Front. Cell Dev. Biol. 2019, 7, 212. [Google Scholar] [CrossRef]





| Group | Total Animals | No. (%) of Mice Challenged with HSV-2 and Virus-Shedding Incidences | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 15 | ||
| pcDNA3.1 | 15 | 15 (100.0) | 15 (100.0) | 15 (100.0) | − | − | − | − |
| pgB + pcDNA3.1 | 15 | 15 (100.0) | 15 (100.0) | 12 (80.0) | 9 (60.0) | ND | ND | 3 (20.0) |
| pgB + pCCL28 | 15 | 15 (100.0) | 9 (60.0) | 5 (33.3) | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Hu, K.; Fu, M.; Deng, X.; Guan, X.; Luo, S.; Zhang, M.; Liu, Y.; Hu, Q. CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice. Vaccines 2022, 10, 1291. https://doi.org/10.3390/vaccines10081291
Yan Y, Hu K, Fu M, Deng X, Guan X, Luo S, Zhang M, Liu Y, Hu Q. CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice. Vaccines. 2022; 10(8):1291. https://doi.org/10.3390/vaccines10081291
Chicago/Turabian StyleYan, Yan, Kai Hu, Ming Fu, Xu Deng, Xinmeng Guan, Sukun Luo, Mudan Zhang, Yalan Liu, and Qinxue Hu. 2022. "CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice" Vaccines 10, no. 8: 1291. https://doi.org/10.3390/vaccines10081291
APA StyleYan, Y., Hu, K., Fu, M., Deng, X., Guan, X., Luo, S., Zhang, M., Liu, Y., & Hu, Q. (2022). CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice. Vaccines, 10(8), 1291. https://doi.org/10.3390/vaccines10081291







