Cervical Staphylococcus aureus Infection after Receiving the Third Dose of COVID-19 Vaccination: A Case Report
Abstract
:1. Introduction
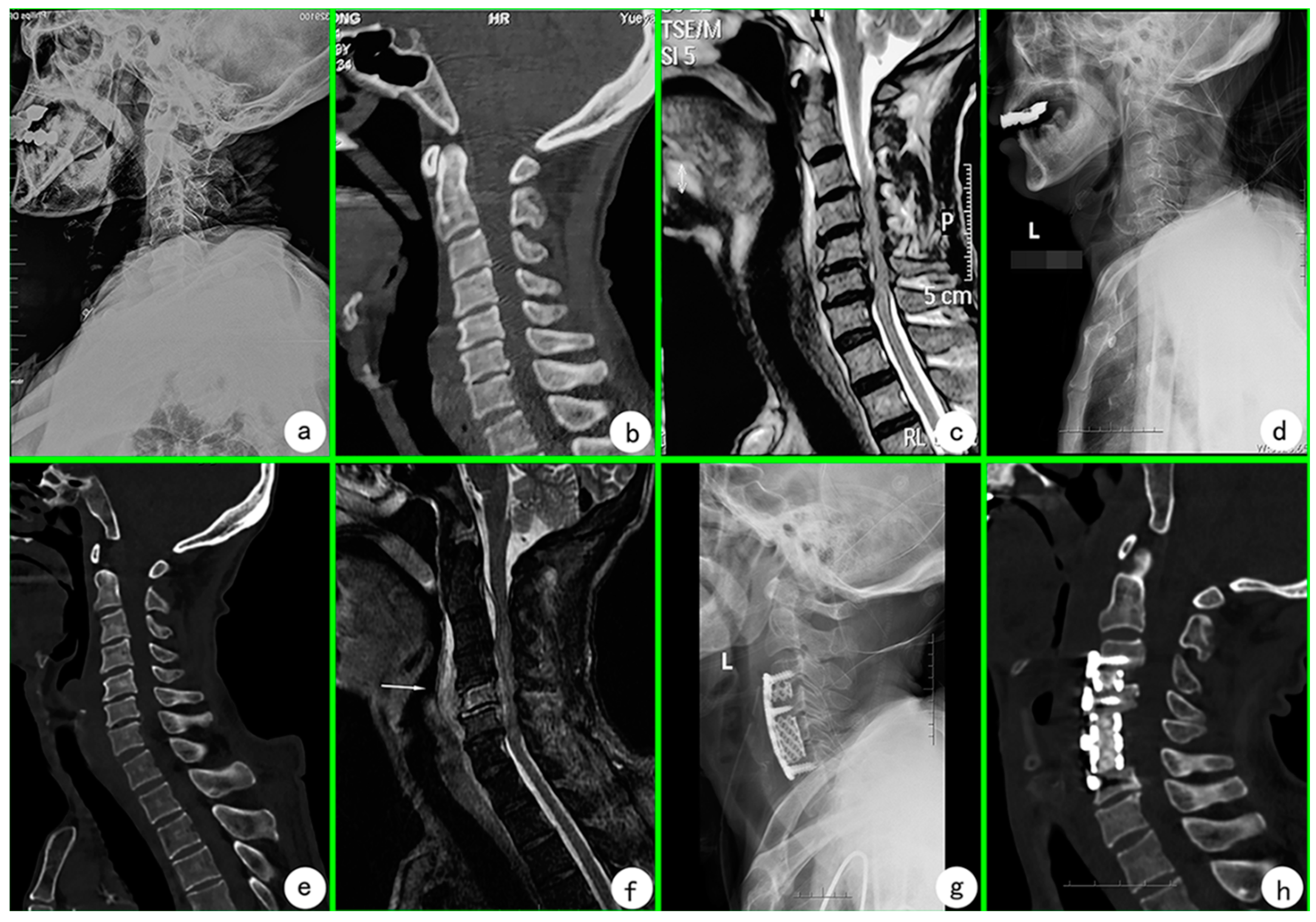
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Rubin, E.J.; Longo, D.L. SARS-CoV-2 Vaccination—An Ounce (Actually, Much Less) of Prevention. N. Engl. J. Med. 2020, 383, 2677–2678. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol. Scand. 2022, 145, 5–9. [Google Scholar] [CrossRef]
- Tozzi, A.E.; Asturias, E.J.; Balakrishnan, M.R.; Halsey, N.A.; Law, B.; Zuber, P.L. Assessment of causality of individual adverse events following immunization (AEFI): A WHO tool for global use. Vaccine 2013, 31, 5041–5046. [Google Scholar] [CrossRef]
- Yee, D.K.; Samartzis, D.; Wong, Y.W.; Luk, K.D.; Cheung, K.M. Infective spondylitis in Southern Chinese: A descriptive and comparative study of ninety-one cases. Spine 2010, 35, 635–641. [Google Scholar] [CrossRef]
- Mylona, E.; Samarkos, M.; Kakalou, E.; Fanourgiakis, P.; Skoutelis, A. Pyogenic vertebral osteomyelitis: A systematic review of clinical characteristics. Semin. Arthritis Rheum. 2009, 39, 10–17. [Google Scholar] [CrossRef]
- Li, T.; Gao, Q.; Guo, C.; Li, Y. Case Report: Diagnosis of Primary Klebsiella pneumoniae in Cervical Spine by Metagenomic Next-Generation Sequencing. Front. Surg. 2022, 9, 800396. [Google Scholar] [CrossRef]
- Gasbarrini, A.L.; Bertoldi, E.; Mazzetti, M.; Fini, L.; Terzi, S.; Gonella, F.; Mirabile, L.; Barbanti Bròdano, G.; Furno, A.; Gasbarrini, A.; et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 53–66. [Google Scholar]
- Babic, M.; Simpfendorfer, C.S. Infections of the Spine. Infect. Dis. Clin. N. Am. 2017, 31, 279–297. [Google Scholar] [CrossRef]
- Quinones-Hinojosa, A.; Jun, P.; Jacobs, R.; Rosenberg, W.S.; Weinstein, P.R. General principles in the medical and surgical management of spinal infections: A multidisciplinary approach. Neurosurg. Focus 2004, 17, E1. [Google Scholar] [CrossRef] [Green Version]
- Hadjipavlou, A.G.; Mader, J.T.; Necessary, J.T.; Muffoletto, A.J. Hematogenous pyogenic spinal infections and their surgical management. Spine 2000, 25, 1668–1679. [Google Scholar]
| Date | WBC (×109/L) | NEUT% | CRP (mg/L) | ESR (mm/h) | PCT (ng/mL) |
|---|---|---|---|---|---|
| 29 March 2022 (D6) | 12.1 | 89.2 | 100.0 | 53 | 0.45 |
| 5 April 2022 (D13) | 14.1 | 85.5 | - | 67 | 0.16 |
| 9 April 2022 (D17) | 7.8 | 74.9 | 93.3 | - | 0.30 |
| 12 April 2022 (S1) | 8.3 | 90.4 | 49.2 | 41 | 0.14 |
| 15 April 2022 (S4) | 7.4 | 75.6 | 35.3 | - | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhang, H.; Wang, Y.; Wang, Y.; Gao, Q.; Tang, M.; Liu, S.; Zhang, G.; Guo, C. Cervical Staphylococcus aureus Infection after Receiving the Third Dose of COVID-19 Vaccination: A Case Report. Vaccines 2022, 10, 1276. https://doi.org/10.3390/vaccines10081276
Li T, Zhang H, Wang Y, Wang Y, Gao Q, Tang M, Liu S, Zhang G, Guo C. Cervical Staphylococcus aureus Infection after Receiving the Third Dose of COVID-19 Vaccination: A Case Report. Vaccines. 2022; 10(8):1276. https://doi.org/10.3390/vaccines10081276
Chicago/Turabian StyleLi, Tao, Hongqi Zhang, Yuxiang Wang, Yunjia Wang, Qile Gao, Mingxing Tang, Shaohua Liu, Gengming Zhang, and Chaofeng Guo. 2022. "Cervical Staphylococcus aureus Infection after Receiving the Third Dose of COVID-19 Vaccination: A Case Report" Vaccines 10, no. 8: 1276. https://doi.org/10.3390/vaccines10081276
APA StyleLi, T., Zhang, H., Wang, Y., Wang, Y., Gao, Q., Tang, M., Liu, S., Zhang, G., & Guo, C. (2022). Cervical Staphylococcus aureus Infection after Receiving the Third Dose of COVID-19 Vaccination: A Case Report. Vaccines, 10(8), 1276. https://doi.org/10.3390/vaccines10081276






