Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Samples Collection
2.3. Data Analysis
3. Results
3.1. Demographics
3.2. Clinical Evaluation
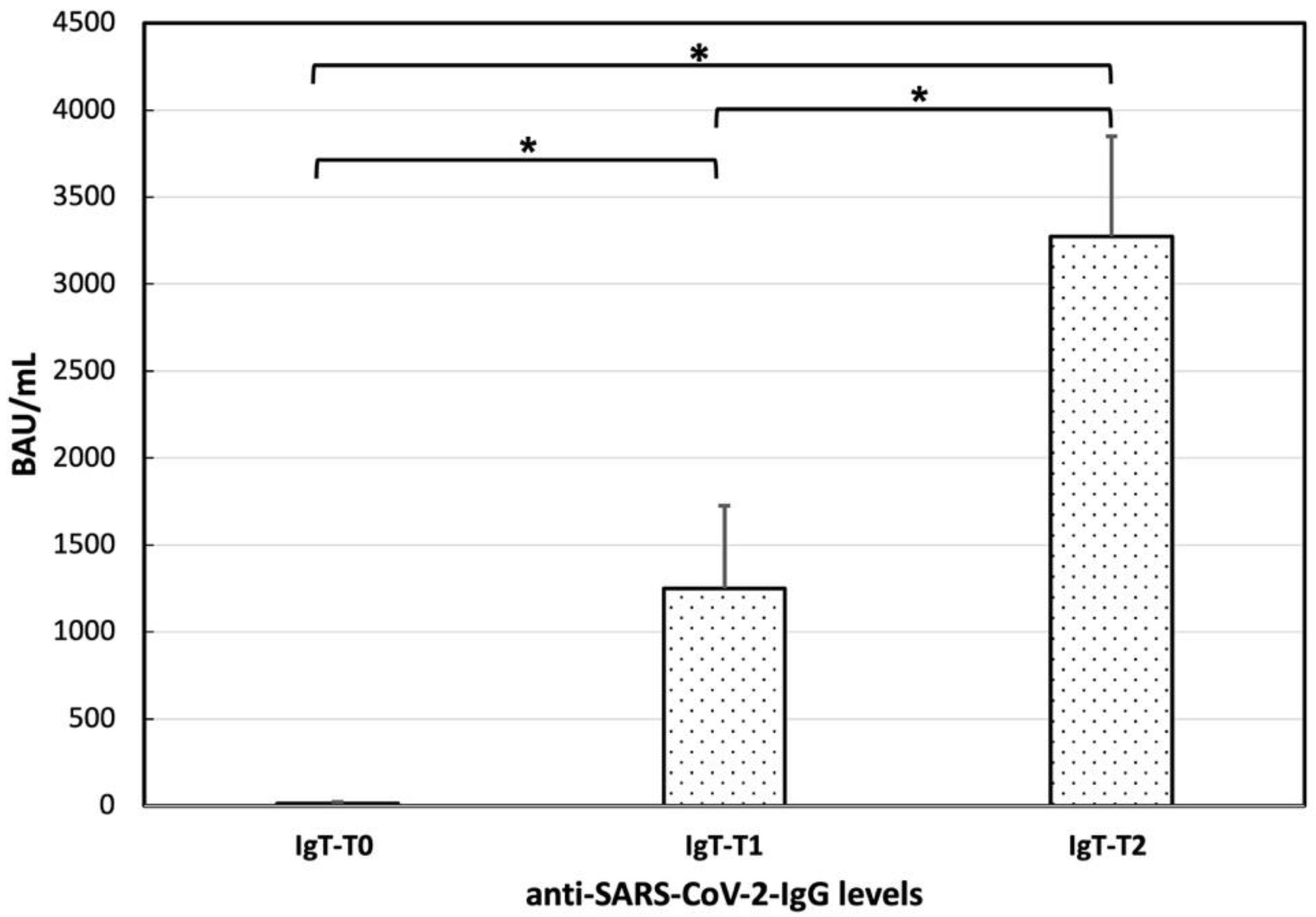
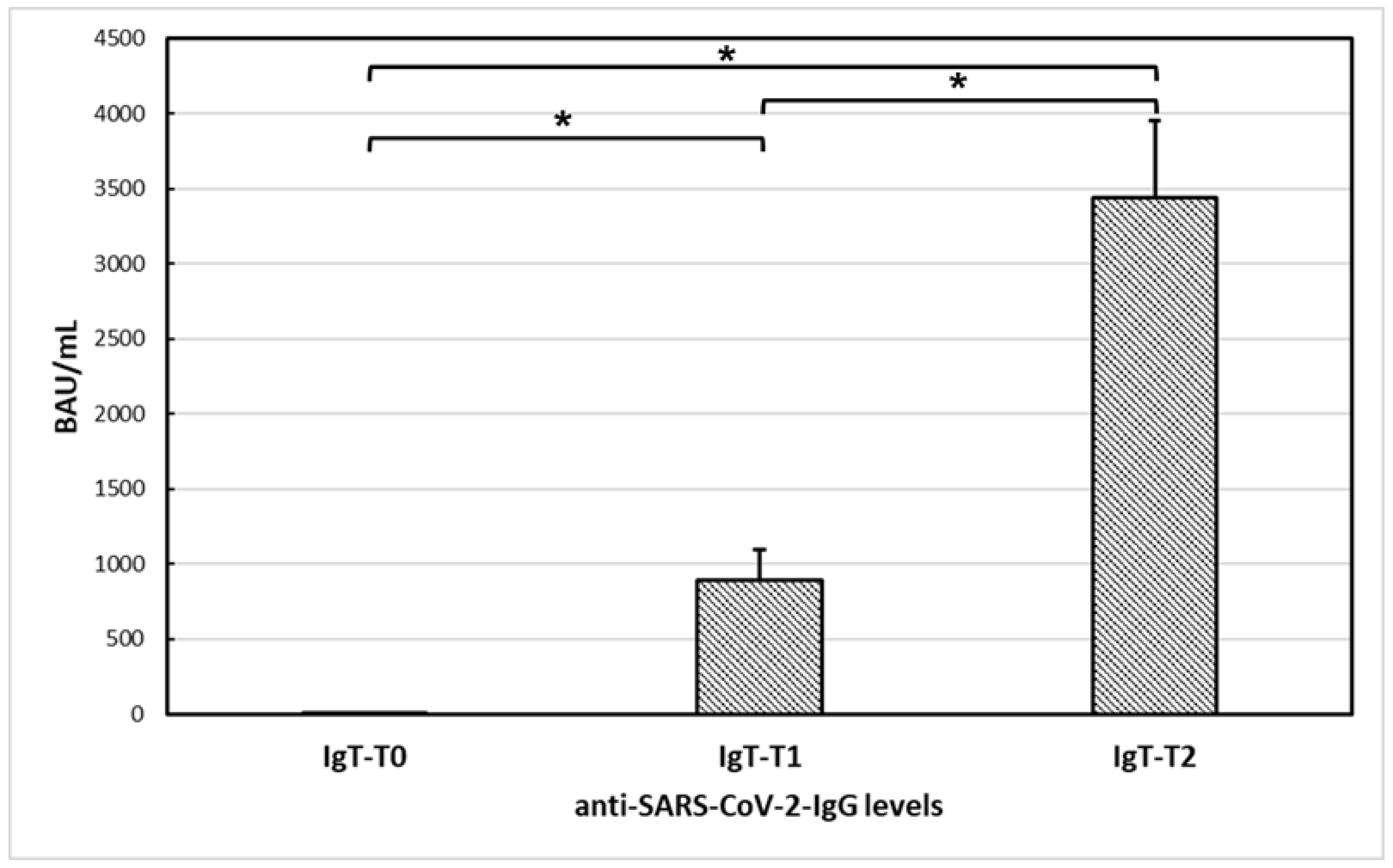
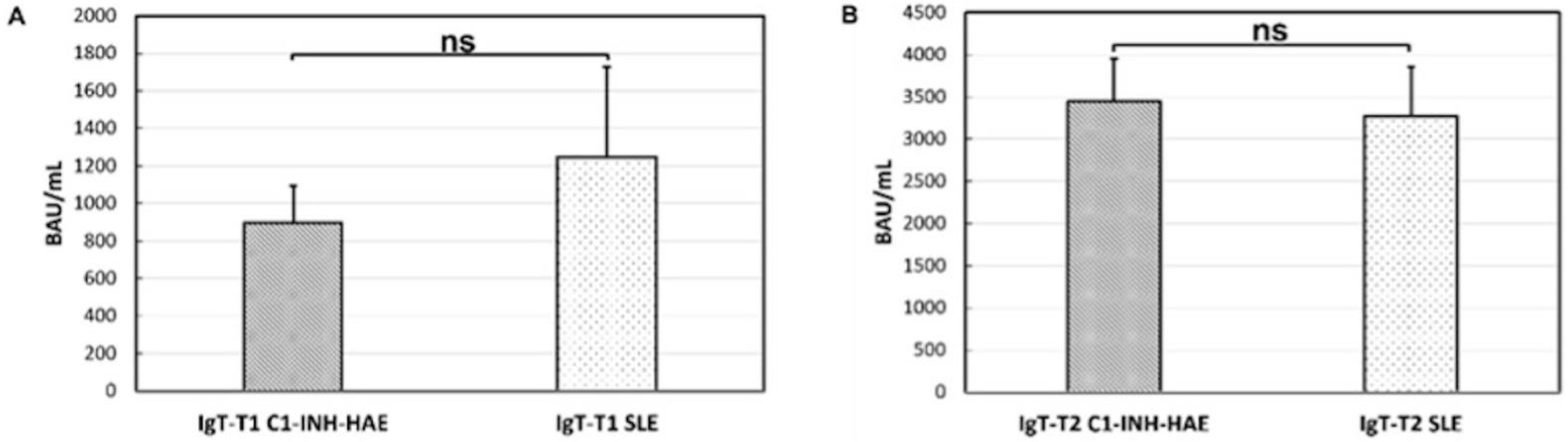
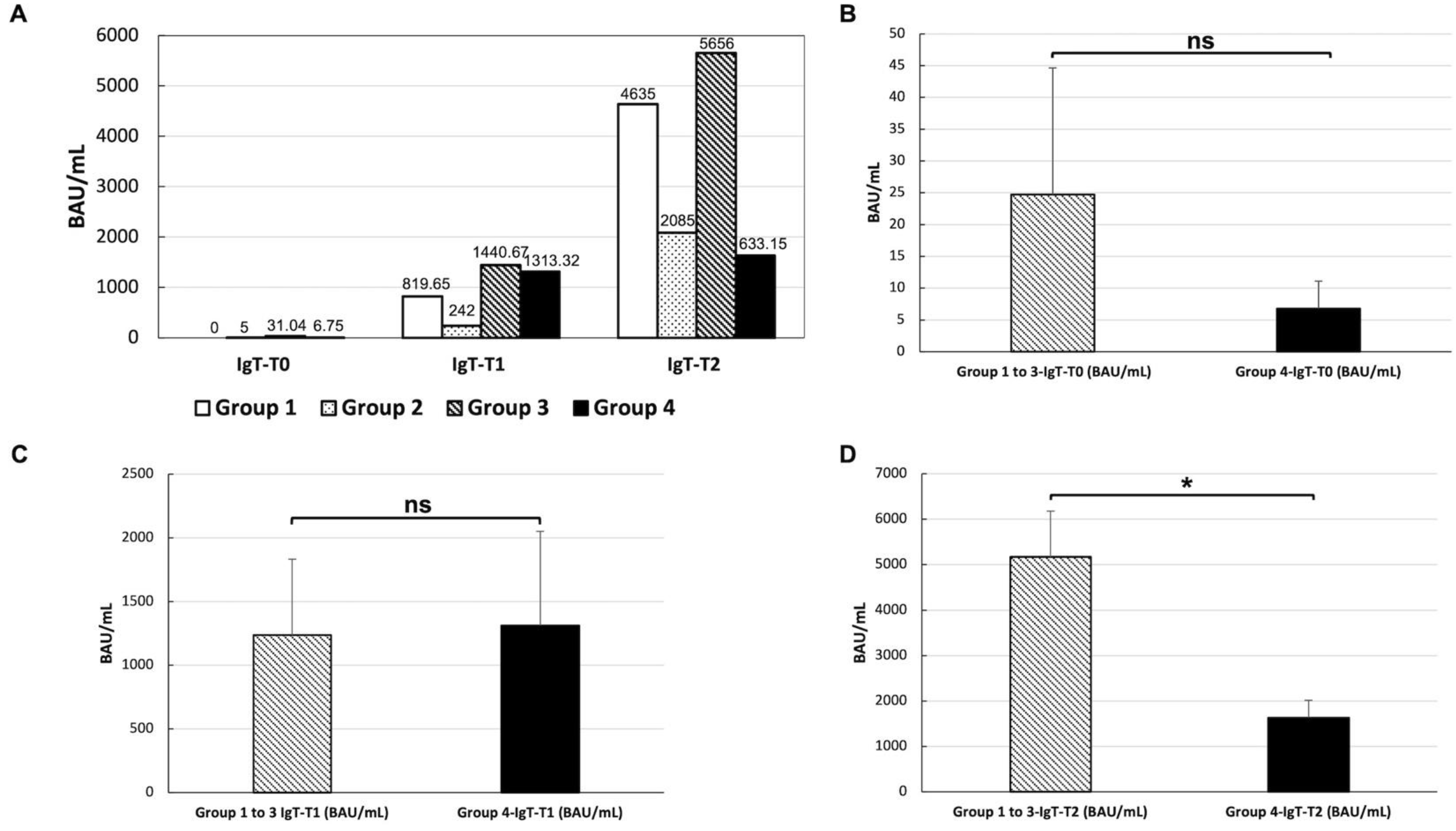
3.3. Anti-SARS-CoV-2 Vaccine Immunogenicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Shoenfeld, Y.; Gershwin, M.E. Surviving the butterfly and the wolf: Mortality trends in systemic lupus erythematosus. Autoimmun. Rev. 2004, 3, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Danza, A.; Ruiz-Irastorza, G. Infection risk in systemic lupus erythematosus patients: Susceptibility factors and preventive strategies. Lupus 2013, 22, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Bermas, B.L.; Petri, M.; Goldman, D.; Mittleman, B.; Miller, M.W.; Stocks, N.I.; Via, C.S.; Shearer, G.M. T helper cell dysfunction in systemic lupus erythematosus (SLE): Relation to disease activity. J. Clin. Immunol. 1994, 14, 169–177. [Google Scholar] [CrossRef]
- Cuchacovich, R.; Gedalia, A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum. Dis. Clin. 2009, 35, 75–93. [Google Scholar] [CrossRef]
- Ho, A.; Barr, S.G.; Magder, L.S.; Petri, M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2350–2357. [Google Scholar] [CrossRef]
- Juarez, M.; Misischia, R.; Alarcon, G.S. Infections in systemic connective tissue diseases: Systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Rheum. Dis. Clin. 2003, 29, 163–184. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, 1–48. [Google Scholar] [CrossRef]
- Thanou, A.; Sawalha, A.H. SARS-CoV-2 and Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Manzi, S. Coronavirus Disease-2019: Implication for the care and management of patients with systemic lupus erythematosus. Eur. J. Rheumatol. 2020, 7, S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.; Hyrich, K.L.; Al-Adely, S.; Carmona, L.; Danila, M.I.; Gossec, L.; Izadi, Z.; Jacobsohn, L.; Katz, P.; Lawson-Tovey, S.; et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020, 79, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B. Interferon alpha as a primary pathogenic factor in human lupus. J. Interferon Cytokine Res. 2011, 31, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzalla Cassione, E.; Zanframundo, G.; Biglia, A.; Codullo, V.; Montecucco, C.; Cavagna, L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann. Rheum. Dis. 2020, 79, 1382–1383. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Patil, P.; Pathak, H.; Santhanam, S.; Goel, A.; Sharma, V.; Pandey, A.; Gupta, N.; Jain, R.; Akerkar, S.; et al. Impact of COVID-19 pandemic on patients with SLE: Results of a large multicentric survey from India. Ann. Rheum. Dis. 2021, 80, e71. [Google Scholar] [CrossRef]
- Strangfeld, A.; Schafer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef]
- So, H.; Li, T.; Chan, V.; Tam, L.S.; Chan, P.K. Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221089586. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sumbul, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021, 80, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [Green Version]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Thanou, A.; James, J.A.; Arriens, C.; Aberle, T.; Chakravarty, E.; Rawdon, J.; Stavrakis, S.; Merrill, J.T.; Askanase, A. Scoring systemic lupus erythematosus (SLE) disease activity with simple, rapid outcome measures. Lupus Sci. Med. 2019, 6, e000365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyon, J.P.; Petri, M.A.; Kim, M.Y.; Kalunian, K.C.; Grossman, J.; Hahn, B.H.; Merrill, J.T.; Sammaritano, L.; Lockshin, M.; Alarcon, G.S.; et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann. Intern. Med. 2005, 142, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Izmirly, P.M.; Kim, M.Y.; Samanovic, M.; Fernandez-Ruiz, R.; Ohana, S.; Deonaraine, K.K.; Engel, A.J.; Masson, M.; Xie, X.; Cornelius, A.R.; et al. Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS-CoV-2 Vaccination. Arthritis Rheumatol. 2022, 74, 284–294. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Fagni, F.; Kronke, G.; Kleyer, A.; Meder, C.; Atreya, R.; Leppkes, M.; Kremer, A.E.; Ramming, A.; et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2021, 80, 1312–1316. [Google Scholar] [CrossRef]
- Braun-Moscovici, Y.; Kaplan, M.; Braun, M.; Markovits, D.; Giryes, S.; Toledano, K.; Tavor, Y.; Dolnikov, K.; Balbir-Gurman, A. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021, 80, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.S.; Yuki, E.F.N.; Pedrosa, T.; Fusco, S.R.G.; Rojo, P.T.; Pereira, R.M.R.; Shinjo, S.K.; Andrade, D.C.O.; et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: A phase 4 trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Assawasaksakul, T.; Sathitratanacheewin, S.; Vichaiwattana, P.; Wanlapakorn, N.; Poovorawan, Y.; Kittanamongkolchai, W. Immunogenicity, safety and reactogenicity of a heterogeneous booster following the CoronaVac inactivated SARS-CoV-2 vaccine in patients with SLE: A case series. RMD Open 2021, 7. [Google Scholar] [CrossRef]
- Yuki, E.F.N.; Borba, E.F.; Pasoto, S.G.; Seguro, L.P.; Lopes, M.; Saad, C.G.S.; Medeiros-Ribeiro, A.C.; Silva, C.A.; de Andrade, D.C.O.; Kupa, L.V.K.; et al. Impact of Distinct Therapies on Antibody Response to SARS-CoV-2 Vaccine in Systemic Lupus Erythematosus. Arthritis Care Res. 2022, 74, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Moyon, Q.; Sterlin, D.; Miyara, M.; Anna, F.; Mathian, A.; Lhote, R.; Ghillani-Dalbin, P.; Breillat, P.; Mudumba, S.; de Alba, S.; et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis 2022, 81, 575–583. [Google Scholar] [CrossRef]
- Nune, A.; Iyengar, K.P.; Ish, P.; Varupula, B.; Musat, C.A.; Sapkota, H.R. The Emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM 2021, 114, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Zafar, S.; Capitle, E.; Khianey, R. COVID-19 Vaccination as a Potential Trigger for New-Onset Systemic Lupus Erythematosus. Cureus 2022, 14, e21917. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Dubois, M.; Ugarte-Gil, M.F.; Chaudier, A.; Kawka, L.; Bergier, H.; Costecalde, C.; Pijnenburg, L.; Fort, J.; Chatelus, E.; et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021, 3, e243–e245. [Google Scholar] [CrossRef]
| Number | Percentage | ||
|---|---|---|---|
| Sex | M | 5 | 12.19% |
| F | 36 | 87.8% | |
| COVID-19 disease | Positive | 32 | 78% |
| Negative | 6 | 14.63% | |
| Unknown | 3 | 7.31% | |
| Antiphospholipids | Positive | 13 | 31.7% |
| Negative | 18 | 43.9% | |
| Unknown | 10 | 24.39% | |
| Immunosuppression level | No therapy | 2 | 4.87% |
| Mild | 2 | 4.87% | |
| Moderate | 15 | 36.58% | |
| High | 22 | 53.66% | |
| Disease activity T0 (SLEDAI) | Inactive disease (0) | 12 | 29.27% |
| Mildly active disease (1–5) | 7 | 17.07% | |
| Moderate actively (6–10) | 6 | 14.63% | |
| Active disease (11–19) | 7 | 17.07% | |
| Very active disease (≥20) | 1 | 2.43% | |
| Not available | 8 | 19.51% | |
| Disease activity T2 (SLEDAI) | Inactive disease (0) | 10 | 24.39% |
| Mildly active disease (1–5) | 9 | 21.95% | |
| Moderate actively (6–10) | 5 | 12.19% | |
| Active disease (11–19) | 3 | 7.32% | |
| Very active disease (≥20) | 1 | 2.43% | |
| Unknown | 13 | 31.7% |
| Treatment | Number of Patients (%) Number of Studied Patients = 41 |
|---|---|
| Patient without therapy | 2 (4.87%) |
| HCQ | 2 (4.87%) |
| PDN (<20 mg/day) | 4 (9.75%) |
| MMF | 1 (2.44%) |
| BLM | 1 (2.44%) |
| HCQ + PDN (<20 mg/day) | 8 (19.5%) |
| HCQ + AZA | 1 (2.44%) |
| HCQ + MTX | 1 (2.44%) |
| HCQ + MMF | 2 (4.87%) |
| HCQ + BLM | 2 (4.87%) |
| PDN (<20 mg/day) + MMF | 2 (4.87%) |
| PDN (<20 mg/day) + CYA | 1 (2.44%) |
| PDN (>20 mg/day) + MMF | 1 (2.44%) |
| HCQ + PDN (<20 mg/day) + BLM | 5 (12.19%) |
| HCQ + PDN (<20 mg/day) + MMF | 5 (12.19%) |
| HCQ + PDN (<20 mg/day) + SCIG | 1 (2.44%) |
| HCQ + PDN (>20 mg/day) + BLM | 1 (2.44%) |
| MMF + BLM | 1 (2.44%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mormile, I.; Della Casa, F.; Petraroli, A.; Furno, A.; Granata, F.; Portella, G.; Rossi, F.W.; de Paulis, A. Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus. Vaccines 2022, 10, 1221. https://doi.org/10.3390/vaccines10081221
Mormile I, Della Casa F, Petraroli A, Furno A, Granata F, Portella G, Rossi FW, de Paulis A. Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus. Vaccines. 2022; 10(8):1221. https://doi.org/10.3390/vaccines10081221
Chicago/Turabian StyleMormile, Ilaria, Francesca Della Casa, Angelica Petraroli, Alessandro Furno, Francescopaolo Granata, Giuseppe Portella, Francesca Wanda Rossi, and Amato de Paulis. 2022. "Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus" Vaccines 10, no. 8: 1221. https://doi.org/10.3390/vaccines10081221
APA StyleMormile, I., Della Casa, F., Petraroli, A., Furno, A., Granata, F., Portella, G., Rossi, F. W., & de Paulis, A. (2022). Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus. Vaccines, 10(8), 1221. https://doi.org/10.3390/vaccines10081221








