Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Reactogenicity
2.3. Immunogenicity
2.4. Statistical Methods
3. Results
3.1. Participants
3.2. Adverse Drug Reactions
3.3. Immunogenicity
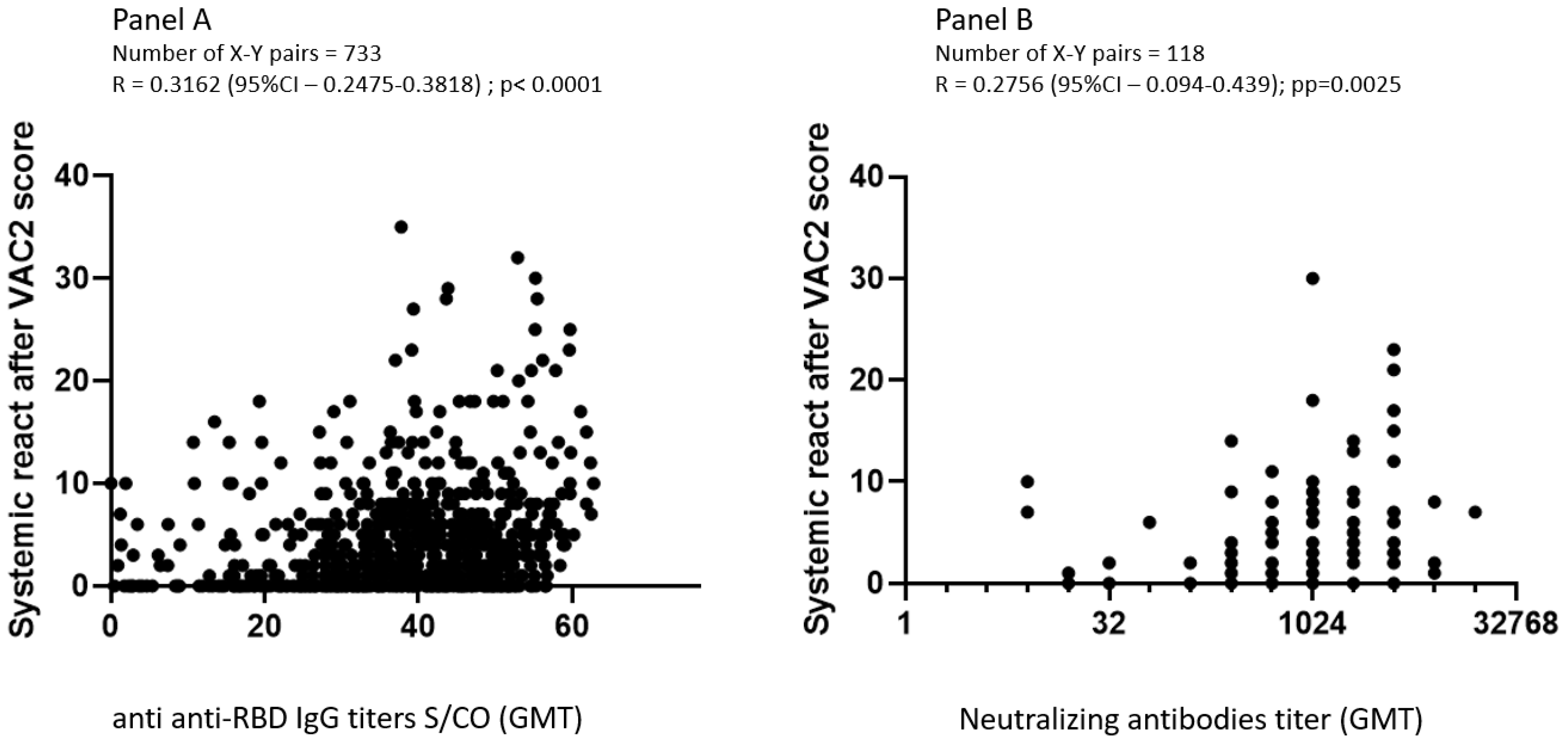
3.4. Correlation between Adverse Effects, Anti-RBD IgG, and Neutralizing Antibodies GMT
3.5. Factors Associated with Adverse Events following Vaccination Using Multivariate Logistic Regression Analysis
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- D’Arminio Monforte, A.; Tavelli, A.; Perrone, P.M.; Za, A.; Razzini, K.; Tomasoni, D.; Bordoni, V.; Romanò, L.; Orfeo, N.; Marchetti, G.; et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3078 health care workers. eClinicalMedicine 2021, 36, 100914. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Koike, R.; Sawahata, M.; Nakamura, Y.; Nomura, Y.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; Sugiyama, K. Systemic Adverse Effects Induced by the BNT162b2 Vaccine Are Associated with Higher Antibody Titers from 3 to 6 Months after Vaccination. Vaccines 2022, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Shimazu, Y.; Kawamura, T.; Nishikawa, Y.; Omata, F.; Kaneko, Y.; Kodama, T.; Tsubokura, M. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLoS ONE 2022, 17, e0269917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Leung, K.-Y.; Liu, D.; Fan, Y.; Lu, L.; Chan, P.-C.; To, K.K.-W.; Chen, H.; Yuen, K.-Y.; Chan, K.-H.; et al. Correlation of Immunogenicity and Reactogenicity of BNT162b2 and CoronaVac SARS-CoV-2 Vaccines. mSphere 2022, 7, e0091521. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; Esse, J.; Tascilar, K.; Steininger, P.; Schober, K.; Irrgang, P.; Alsalameh, R.; Tenbusch, M.; Seggewies, C.; Bogdan, C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®). Vaccines 2021, 9, 1063. [Google Scholar] [CrossRef]
- Lustig, Y.; Nemet, I.; Kliker, L.; Zuckerman, N.; Yishai, R.; Alroy-Preis, S.; Mendelson, E.; Mandelboim, M. Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2. N. Engl. J. Med. 2021, 384, 2453–2454. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, K.-H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021, 36, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Burny, W.; Hervé, C.; Kim, J.H.; Levin, M.J.; Zahaf, T.; Cunningham, A.L.; Didierlaurent, A.M. Association between Immunogenicity and Reactogenicity: A Post Hoc Analysis of 2 Phase 3 Studies with the Adjuvanted Recombinant Zoster Vaccine. J. Infect. Dis. 2021, 20, jiab536. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Lan, R.; Sun, N.; Wu, M.; Joung, S.; Botwin, G.J.; Botting, P.; Al-Amili, D.; Aronow, H.; Beekley, J.; et al. Symptomology following mRNA vaccination against SARS-CoV-2. Prev. Med. 2021, 153, 106860. [Google Scholar] [CrossRef]
- Hervé, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Da Silva, F.T. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saita, M.; Yan, Y.; Ito, K.; Sasano, H.; Seyama, K.; Naito, T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers in Japan. J. Infect. Chemother. 2022, 28, 116–119. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Enders, P.; Buchholz, H.-G.; Plachter, B. Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany. Vaccines 2021, 9, 911. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.G.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130, Erratum in Lancet 2021, 398, 582. [Google Scholar] [CrossRef]
- Powell, A.A.; Power, L.; Westrop, S.; McOwat, K.; Campbell, H.; Simmons, R.; Ramsay, M.E.; Brown, K.; Ladhani, S.N.; Amirthalingam, G. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Eurosurveillance 2021, 26, 2100634. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Pietrobon, A.J.; Teixeira, F.M.E.; Sato, M.N. I mmunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front. Immunol. 2020, 11, 579220. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Fang, F.; Weyand, C.M.; Goronzy, J.J. The life cycle of a T cell after vaccination—Where does immune ageing strike? Clin. Exp. Immunol. 2017, 187, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancro, M.P. Age-Associated B Cells. Annu. Rev. Immunol. 2020, 38, 315–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferson, T.; Rivetti, D.; Rivetti, A.; Rudin, M.; Di Pietrantonj, C.; Demicheli, V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet 2005, 366, 1165–1174, Erratum in Lancet 2006, 367, 986. [Google Scholar] [CrossRef]
- Djennad, A.; Ramsay, M.E.; Pebody, R.; Fry, N.K.; Sheppard, C.; Ladhani, S.N.; Andrews, N.J. Effectiveness of 23-Valent Polysaccharide Pneumococcal Vaccine and Changes in Invasive Pneumococcal Disease Incidence from 2000 to 2017 in Those Aged 65 and over in England and Wales. eClinicalMedicine 2019, 6, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L.; Pekosz, A. Sex-based Biology and the Rational Design of Influenza Vaccination Strategies. J. Infect. Dis. 2014, 209 (Suppl. 3), S114–S119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [Green Version]

| Adverse Event | Number of Points for Each Day of the Event |
|---|---|
| Fatigue | 1 |
| Headache | 1 |
| Myalgia | 2 |
| Fever > 38 °C | 2 |
| Arthralgia | 1 |
| Local Lymphadenopathy | 1 |
| Systemic rash | 1 |
| Pruritus | 1 |
| Facial paresthesia | 1 |
| Non-facial paresthesia | 1 |
| Need for antipyretic or analgesic medication | 2 |
| Total score | Sum of the above |
| Adverse Event | After 1st Dose N = 831 (100%) | After 2nd Dose N = 738 (100%) | p * |
|---|---|---|---|
| At least one adverse event | 711 (85.6) | 673 (91.2) | 0.0003 |
| Any local AE | 693 (83.4) | 615 (83.3) | 0.5807 |
| Pain at injection site | 693 (83.4) | 614 (83.2) | 0.5279 |
| Heat at injection site | 61 (7.3) | 62 (8.4) | 0.4855 |
| Local erythema | 40 (4.8) | 50 (6.8) | 0.1229 |
| Any systemic AE | 274 (33.0) | 614 (83.2) | <0.0001 |
| Axillary Lymphadenopathy | 8 (1.0) | 48 (6.5) | <0.0001 |
| Fever > 38 °C | 5 (0.6) | 53 (7.2) | <0.0001 |
| Fatigue | 174 (20.9) | 359 (48.6) | <0.0001 |
| Myalgia | 95 (11.4) | 274 (37.1) | <0.0001 |
| Headache | 125 (15.0) | 279 (37.8) | <0.0001 |
| Arthralgia | 23 (2.8) | 105 (14.2) | <0.0001 |
| Throat pain | 22 (2.6) | 35 (4.7) | 0.0159 |
| Facial paresthesia | 9 (1.1) | 9 (1.2) | 0.7963 |
| Non-facial paresthesia | 9 (1.1) | 23 (3.1) | 0.0025 |
| Pruritus | 6 (0.7) | 17 (2.3) | 0.0105 |
| Systemic AEs total Score—median (Q1–Q3) | 0 (0–2) | 2.5 (0–6) | <0.0001 |
| Missing 1 workday | 2 (0.2) | 53 (7.2) | <0.0001 |
| Missing 2 + workdays | 2 (0.2) | 26 (3.5) | <0.0001 |
| Need for anti-fever or anti-pain medication | 86 (10.3) | 222 (30.1) | <0.0001 |
| Assay | After 1st Vaccine * | After 2nd Vaccine ** |
|---|---|---|
| Days for Anti-RBD–IgG; mean ± SD (range) | 11.7 ± 3.6 (7–21) | 13.7 ± 5.6 days (7–38) |
| Anti-RBD-IgG (Geometric mean (CI95%)) | 0.11 (0.08–0.14) | 32.55 (31.11–34.05) |
| Days for neutralizing Ab; Mean ± SD (range) | 14.1 ± 0.4 (13–17) | 6.9 ± 0.6 (4–10) |
| Neutralizing Ab (Geometric mean (CI95%)) | 23.41 (17.95–30.54) | 745 (611.5–908.9) |
| Anti-IgG-RBD | Neutralizing AB | |||||||
|---|---|---|---|---|---|---|---|---|
| After 1st Vaccination | After 2nd Vaccination | After 1st Vaccination | After 2nd Vaccination | |||||
| N | 233 | 733 | 89 | 118 | ||||
| R | p | R | p | R | p | R | p | |
| Score of systemic AEs | 0.1 | 0.13 | 0.366 | <0.0001 | −0.1 | 0.34 | 0.283 | 0.005 |
| Axillary lymphadenopathy | −0.018 | 0.78 | 0.15 | <0.0001 | NA | 0.1 | 0.28 | |
| Fever | 0.12 | 0.7 | 0.19 | <0.0001 | −0.2 | 0.057 | 0.13 | 0.15 |
| Fatigue | 0.1 | 0.12 | 0.18 | <0.0001 | −0.08 | 0.46 | 0.18 | 0.04 |
| Headache | 0.07 | 0.04 | 0.19 | <0.0001 | 0.08 | 0.45 | 0.12 | 0.18 |
| Myalgia | 0.14 | 0.3 | 0.21 | <0.0001 | 0.017 | 0.87 | 0.2 | 0.03 |
| Arthralgia | 0.09 | 0.15 | 0.135 | <0.05 | 0.003 | 0.97 | 0.03 | 0.7 |
| Need for antipyretic or analgetic medication | 0.078 | 0.24 | 0.197 | <0.0001 | −0.12 | 0.25 | −0.16 | 0.07 |
| Missing workdays | 0.06 | 0.36 | 0.11 | 0.0027 | NA | 0.06 | 0.4 | |
| After 1st Vaccination (N = 233) C Statistics = 0.719 | After 2nd Vaccination (N = 733) C Statistics = 0.751 | |||
|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | |
| Female | 2.38 (1.02–5.55) | 0.0441 | 2.86 (1.6–5.1) | 0.0004 |
| Age < 55 years old | 3.29 (1.47–7.39) | 0.0039 | 3.18 (1.83–5.52) | <0.0001 |
| IgG-Anti-RBD | 1.15 (0.88–1.51) | 0.3 | 1.36 (1.33–1.39) | 0.0029 |
| Number of days after second vaccine | 0.96 (0.86–1.08) | 0.5143 | 1.01 (0.96–1.06) | 0.6905 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, I.; Levin, E.G.; Olmer, L.; Regev-Yochay, G.; Agmon-Levin, N.; Wieder-Finesod, A.; Indenbaum, V.; Herzog, K.; Doolman, R.; Asraf, K.; et al. Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine. Vaccines 2022, 10, 1220. https://doi.org/10.3390/vaccines10081220
Levy I, Levin EG, Olmer L, Regev-Yochay G, Agmon-Levin N, Wieder-Finesod A, Indenbaum V, Herzog K, Doolman R, Asraf K, et al. Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine. Vaccines. 2022; 10(8):1220. https://doi.org/10.3390/vaccines10081220
Chicago/Turabian StyleLevy, Itzchak, Einav Gal Levin, Liraz Olmer, Gili Regev-Yochay, Nancy Agmon-Levin, Anat Wieder-Finesod, Victoria Indenbaum, Karin Herzog, Ram Doolman, Keren Asraf, and et al. 2022. "Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine" Vaccines 10, no. 8: 1220. https://doi.org/10.3390/vaccines10081220
APA StyleLevy, I., Levin, E. G., Olmer, L., Regev-Yochay, G., Agmon-Levin, N., Wieder-Finesod, A., Indenbaum, V., Herzog, K., Doolman, R., Asraf, K., Halperin, R., Lustig, Y., & Rahav, G. (2022). Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine. Vaccines, 10(8), 1220. https://doi.org/10.3390/vaccines10081220








