Lymphedema of the Arm after COVID-19 Vaccination in a Patient with Hidden Breast Cancer and Paraneoplastic Dermatomyositis
Abstract
:1. Introduction
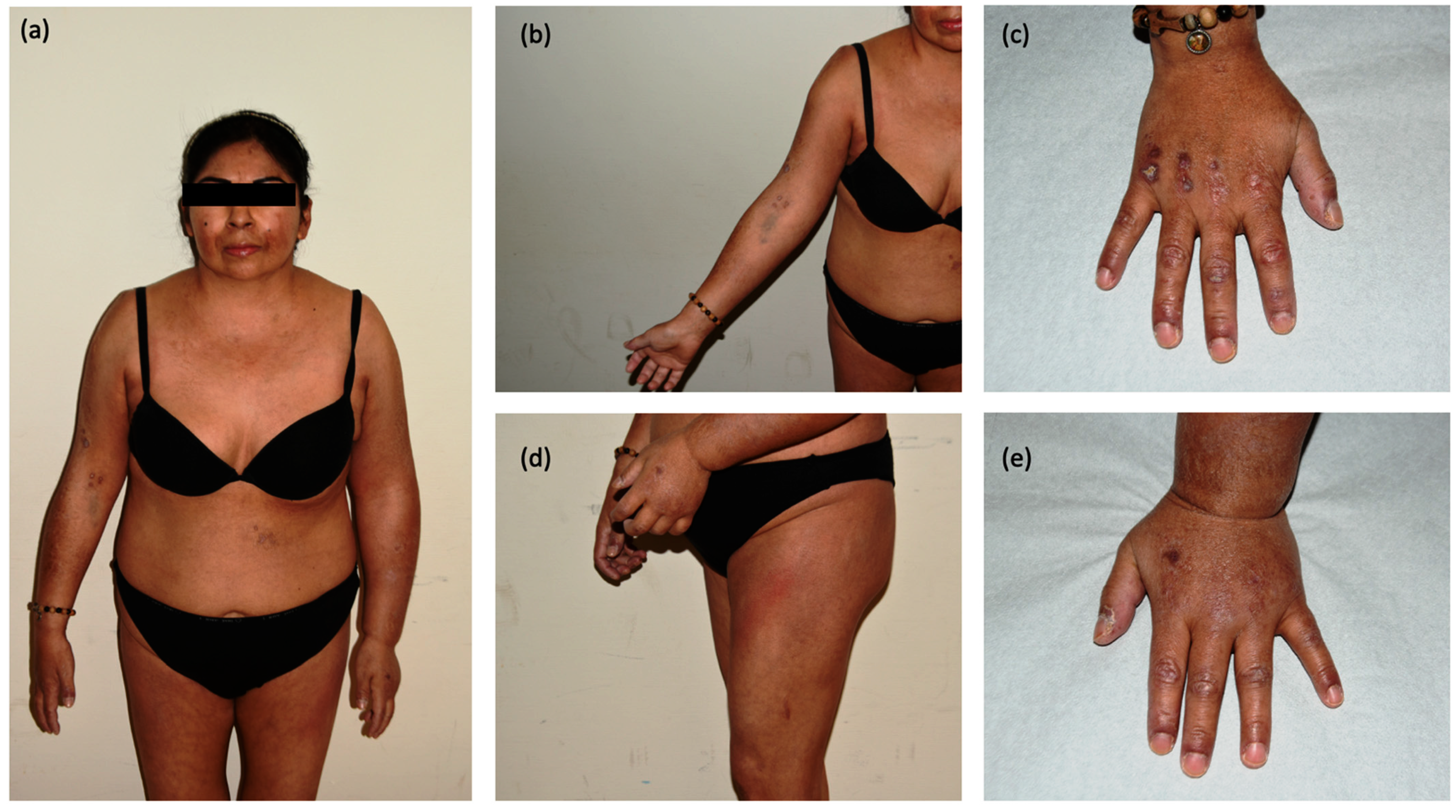
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca (accessed on 18 July 2022).
- Sookaromdee, P.; Wiwanitkit, V. Multisystem inflammatory syndrome and COVID-19 vaccine. Acta Paediatr. 2022, 111, 193. [Google Scholar] [CrossRef] [PubMed]
- Perez Perez, G.I.; Talebi Bezmin Abadi, A. Ongoing challenges faced in the global control of COVID-19 pandemic. Arch. Med. Res. 2020, 51, 574–576. [Google Scholar] [CrossRef]
- Lee, A.C.K.; Morling, J.R. COVID-19 vaccine dilemmas. Public Health 2022, 202, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, J.; Song, T.J. A disproportionality analysis for the association of central nervous system demyelinating diseases with COVID-19 vaccination using the World Health Organization pharmacovigilance database. Mult. Scler. 2022, 13, 13524585221109397. [Google Scholar] [CrossRef]
- Allen-Philbey, K.; Stennett, A.; Begum, T.; Johnson, A.C.; MacDougall, A.; Green, S.; Dobson, R.; Giovannoni, G.; Gnanapavan, S.; Marta, M.; et al. Did it hurt? COVID-19 vaccination experience in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 65, 104022. [Google Scholar] [CrossRef]
- Lupica, A.; Di Stefano, V.; Iacono, S.; Pignolo, A.; Quartana, M.; Gagliardo, A.; Fierro, B.; Brighina, F. Impact of COVID-19 in AChR Myasthenia Gravis and the safety of vaccines: Data from an italian cohort. Neurol. Int. 2022, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Johnson, S.R.; Fraenkel, L.; Arasaratnam, R.J.; Baden, L.R.; Bermas, B.L.; Chatham, W.; Cohen, S.; Costenbader, K.; Gravallese, E.M.; et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: Version 2. Arthritis Rheumatol. 2020, 72, e1–e12. [Google Scholar] [PubMed]
- Gelfand, J.M.; Armstrong, A.W.; Bell, S.; Anesi, G.L.; Blauvelt, A.; Calabrese, C.; Dommasch, E.D.; Feldman, S.R.; Gladman, D.; Kircik, L.; et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 2-advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J. Am. Acad. Dermatol. 2021, 84, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Damiani, A.; Infantino, M.; Manfredi, M.; Lari, B.; Grossi, V.; Mariotti, E.B.; Corrà, A.; Aimo, C.; Quintarelli, L.; et al. Vaccination for SARS-CoV-2 in patients with psoriatic arthritis: Can therapy affect the immunological response? Front. Med. 2022, 288, 11829. [Google Scholar] [CrossRef] [PubMed]
- Laporte, B.E.P.; Laporte, E.G.J.; Aarestrup, P.F.; Aarestrup, M.F.; Aarestrup, F.M. Coronavirus disease 2019 vaccination for cancer patients: Risk or benefit? Rev. Bras. Ginecol. Obstet. 2022, 44, 602–608. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Kassianos, G.; Puig-Barberà, J.; Dinse, H.; Teufel, M.; Türeci, Ö.; Pather, S. Addressing COVID-19 vaccine hesitancy. Drugs Context 2022, 20, 2021-12-3. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Rafiee, F.; Mizandari, M. Lymphadenopathy Following COVID-19 Vaccination: Imaging Findings Review. Acad. Radiol. 2021, 28, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Burger, I.A.; Husmann, L.; Hany, T.F.; Schmid, D.T.; Schaefer, N.G. In-cidence and intensity of F-18 FDG uptake after vaccination withH1N1 vaccine. Clin. Nucl. Med. 2011, 36, 848–853. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech republic. J. Clin. Med. 2021, 1, 1428. [Google Scholar] [CrossRef]
- Hiller, N.; Goldberg, S.N.; Cohen-Cymberknoh, M.; Vainstein, V.; Simanovsky, N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus 2021, 23, e13524. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Sohn, S.M.; Yoo, H.J.; Yoon, E.S.; Park, S.H. Transient lower extremity lymphedema following COVID-19 vaccination: A case report. Medicine 2021, 100, e28092. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: Incidence assessed by [18F] FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854–1863. [Google Scholar] [CrossRef]
- Parodi, A.; Caproni, M.; Marzano, A.V.; De Simone, C.; La Placa, M.; Quaglino, P.; Fornasa, C.V.; Zane, C.; Vaccaro, M.; Papini, M.; et al. Dermatomyositis in 132 patients with different clinical subtypes: Cutaneous signs, constitutional symptoms and circulating antibodies. Acta Derm. Venereol. 2002, 82, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Concha, J.S.S.; Patsatsi, A.; Marshak-Rothstein, A.; Liu, M.L.; Sinha, A.A.; Lee, L.A.; Merola, J.F.; Jabbari, A.; Gudjonsson, J.E.; Chasset, F.; et al. Advances in cutaneous lupus erythematosus and dermatomyositis: A report from the 4th international conference on cutaneous lupus erythematosus-an ongoing need for international consensus and collaborations. J. Investig. Dermatol. 2019, 139, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Findlay, A.R.; Goyal, N.A.; Mozaffar, T. An overview of polymyositis and dermatomyositis. Muscle Nerve 2015, 51, 638–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pectasides, D.; Koumpou, M.; Gaglia, A.; Pectasides, M.; Lambadiari, V.; Lianos, E.; Papaxoinis, G.; Economopoulos, T. Dermatomyositis associated with breast cancer. Anticancer Res. 2006, 26, 2329–2331. [Google Scholar] [PubMed]
- Sieb, J.P. Myasthenia gravis: An update for the clinician. Clin Exp. Immunol. 2022, 175, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Stockton, D.; Doherty, V.R.; Brewster, D.H. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: A Scottish population-based cohort study. Br. J. Cancer 2001, 6, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Cardelli, C.; Zanframundo, G.; Cometi, L.; Marcucci, E.; Biglia, A.; Cavagna, L.; Barsotti, S. Idiopathic inflammatory myopathies: One year in review 2021. Clin. Exp. Rheumatol. 2022, 40, 199–209. [Google Scholar] [CrossRef]
- Yoshida, A.; Ikegami, T.; Igawa, K. Two cases of anti-TIF1-γ antibody positive dermatomyositis with manifested symptoms after SARS-CoV-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e517–e520. [Google Scholar] [CrossRef]
- Kreuter, A.; Lausch, S.; Burmann, S.N.; Paschos, A.; Michalowitz, A.L. Onset of amyopathic dermatomyositis following mRNA-based SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 27, 4–30. [Google Scholar] [CrossRef]
- Stübgen, J.P. A review on the association between inflammatory myopathies and vaccination. Autoimmun. Rev. 2014, 13, 31–39. [Google Scholar] [CrossRef]
- Zhou, W.; DeMartini, W.B.; Ikeda, D.M. Frequency and outcomes of ipsilateral axillary lymphadenopathy after COVID-19 vaccination. JAMA Netw. Open 2022, 5, e2216172. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.A.; Khil, E.K.; Byeon, S.J.; Kang, H.J.; Choi, J.A. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: Case series with a review of literature. Semin Oncol. 2021, 48, 283–291. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, W.; Chen, N.; Li, J.; Wang, X.; Li, M.; Wang, D.; Jiang, L. Misdiagnosis of reactive lymphadenopathy remotely after COVID-19 vaccination: A case report and literature review. Front. Immunol. 2022, 13, 875637. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Akiyama, T.; Ueda, N.; Matsumura, S.; Mori, M.; Namiki, M.; Yamada, N.; Tsutsumi, C.; Tozaki, S.; Iwamoto, H.; et al. COVID-19 Vaccination in patients with cancer. Cancers 2022, 14, 2556. [Google Scholar] [CrossRef] [PubMed]
- Eifer, M.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Shams, J.; Keret, N.; Gorfine, M.; Eshet, Y. COVID-19 mRNA Vaccination: Age and immune status and its association with axillary lymph node PET/CT uptake. J. Nucl. Med. 2022, 63, 134–139. [Google Scholar] [CrossRef]
- Karagiannis, S.N.; Arnold, J.N. Immune cell-antibody interactions in health and disease. Clin. Exp. Immunol. 2022, 26, uxac065. [Google Scholar] [CrossRef] [PubMed]
- Bshesh, K.; Khan, W.; Vattoth, A.L.; Janjua, E.; Nauman, A.; Almasri, M.; Mohamed Ali, A.; Ramadorai, V.; Mushannen, B.; AlSubaie, M.; et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: A systematic review. J. Med. Virol. 2022, 94, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Giorgis, S.; Garlaschi, A.; Brunetti, N.; Tosto, S.; Rescinito, G.; Monetti, F.; Oddone, C.; Massa, B.; Pitto, F.; Calabrese, M.; et al. Axillary adenopathy after COVID-19 vaccine in patients undergoing breast ultrasound. J. Ultrason. 2021, 21, e361–e364. [Google Scholar] [CrossRef]
- McIntosh, L.J.; Bankier, A.A.; Vijayaraghavan, G.R.; Licho, R.; Rosen, M.P. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An emerging dilemma and suggestions for management. AJR Am. J. Roentgenol. 2021, 217, 975–983. [Google Scholar] [CrossRef]
- Shah, S.; Wagner, T.; Nathan, M.; Szyszko, T. COVID-19 vaccine-related lymph node activation—Patterns of uptake on PET-CT. BJR Case Rep. 2021, 7, 20210040. [Google Scholar] [CrossRef]

| Case Report | Clinical Presentation | Cancer | Vaccine | Management |
|---|---|---|---|---|
| 1 61-year-old woman | Multiple, enlarged lymph nodes in left axillary region on imaging studies in a routine 5-year follow-up | Right breast cancer with ipsilateral axillary metastasis | I dose of Vaxzevria in the left arm 16 days before | Biopsy (benign hyperplasia) |
| 2 75-year-old woman | Multiple enlarged, round, and coffee bean-shaped lymph nodes in left axillary region during imaging studies in a routine 2.5-year follow-up | Right breast cancer without axillary metastases | II dose of the Pfizer-BioNTech 14 days before | Biopsy (reactive hyperplasia) |
| 3 71-year-old woman | Smooth and diffuse enlargement of left axillary level I lymph nodes during 8-year surveillance exams | Right breast cancer without axillary metastasis | I dose of Vaxzevria in the left arm 8 and 14 days prior to current CT and US evaluation, respectively | Biopsy (benign hyperplasia) |
| 4 73-year-old woman | Several enlarged lymph nodes in level I of her right axilla with one lymph node showing round shape during 10-year surveillance | Left breast cancer without axillary metastasis | Booster dose Vaxzevria–Pfizer-BioNTech cross-inoculation 28 days before | 4–12 weeks follow-up |
| 5 62-year-old woman | Unilateral left axillary lymphadenopathy during 2.5-year follow-up | Right breast cancer with ipsilateral axillary metastases | I dose of Vaxzevria in the left arm 3 weeks before | 4–12 weeks follow-up |
| 6 61-year-old woman | Right axillary lymphadenopathy detected during 3-year follow-up | Left breast cancer with ipsilateral axillary metastasis | I dose of Vaxzevria in the right arm 19 days before | 3-month follow-up |
| 7 34-year-old woman | Left axillary lymphadenopathy | She denied a medical history of past malignant tumors | II dose of CoronaVac in the left arm 4 months before | Biopsy (reactive hyperplasia) |
| 8 Woman, unknown age | Worsening of lymphedema on the cancer side | Right breast cancer with lymph node dissection | I dose of COVID-19 vaccine in the left arm | Conservative treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimo, C.; Mariotti, E.B.; Corrà, A.; Quintarelli, L.; Bianchi, B.; Verdelli, A.; Ruffo di Calabria, V.; Caproni, M. Lymphedema of the Arm after COVID-19 Vaccination in a Patient with Hidden Breast Cancer and Paraneoplastic Dermatomyositis. Vaccines 2022, 10, 1219. https://doi.org/10.3390/vaccines10081219
Aimo C, Mariotti EB, Corrà A, Quintarelli L, Bianchi B, Verdelli A, Ruffo di Calabria V, Caproni M. Lymphedema of the Arm after COVID-19 Vaccination in a Patient with Hidden Breast Cancer and Paraneoplastic Dermatomyositis. Vaccines. 2022; 10(8):1219. https://doi.org/10.3390/vaccines10081219
Chicago/Turabian StyleAimo, Cristina, Elena Biancamaria Mariotti, Alberto Corrà, Lavinia Quintarelli, Beatrice Bianchi, Alice Verdelli, Valentina Ruffo di Calabria, and Marzia Caproni. 2022. "Lymphedema of the Arm after COVID-19 Vaccination in a Patient with Hidden Breast Cancer and Paraneoplastic Dermatomyositis" Vaccines 10, no. 8: 1219. https://doi.org/10.3390/vaccines10081219
APA StyleAimo, C., Mariotti, E. B., Corrà, A., Quintarelli, L., Bianchi, B., Verdelli, A., Ruffo di Calabria, V., & Caproni, M. (2022). Lymphedema of the Arm after COVID-19 Vaccination in a Patient with Hidden Breast Cancer and Paraneoplastic Dermatomyositis. Vaccines, 10(8), 1219. https://doi.org/10.3390/vaccines10081219






