Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. RBD- and S1-Specific Antibody Titers
2.3. Avidity Assay of Anti-Peptide Antibodies
2.4. Neutralization Assay
2.5. Statistics
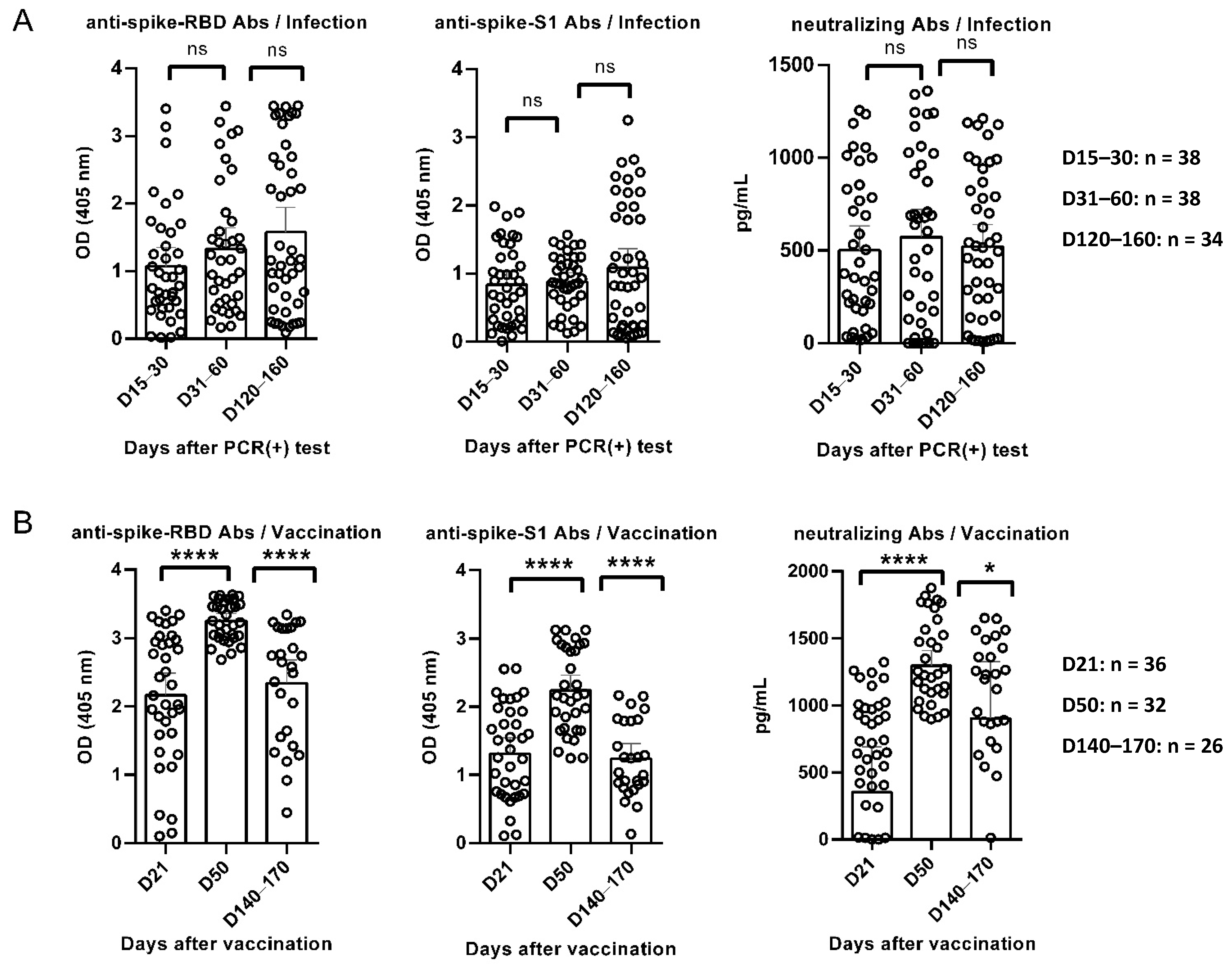
3. Results
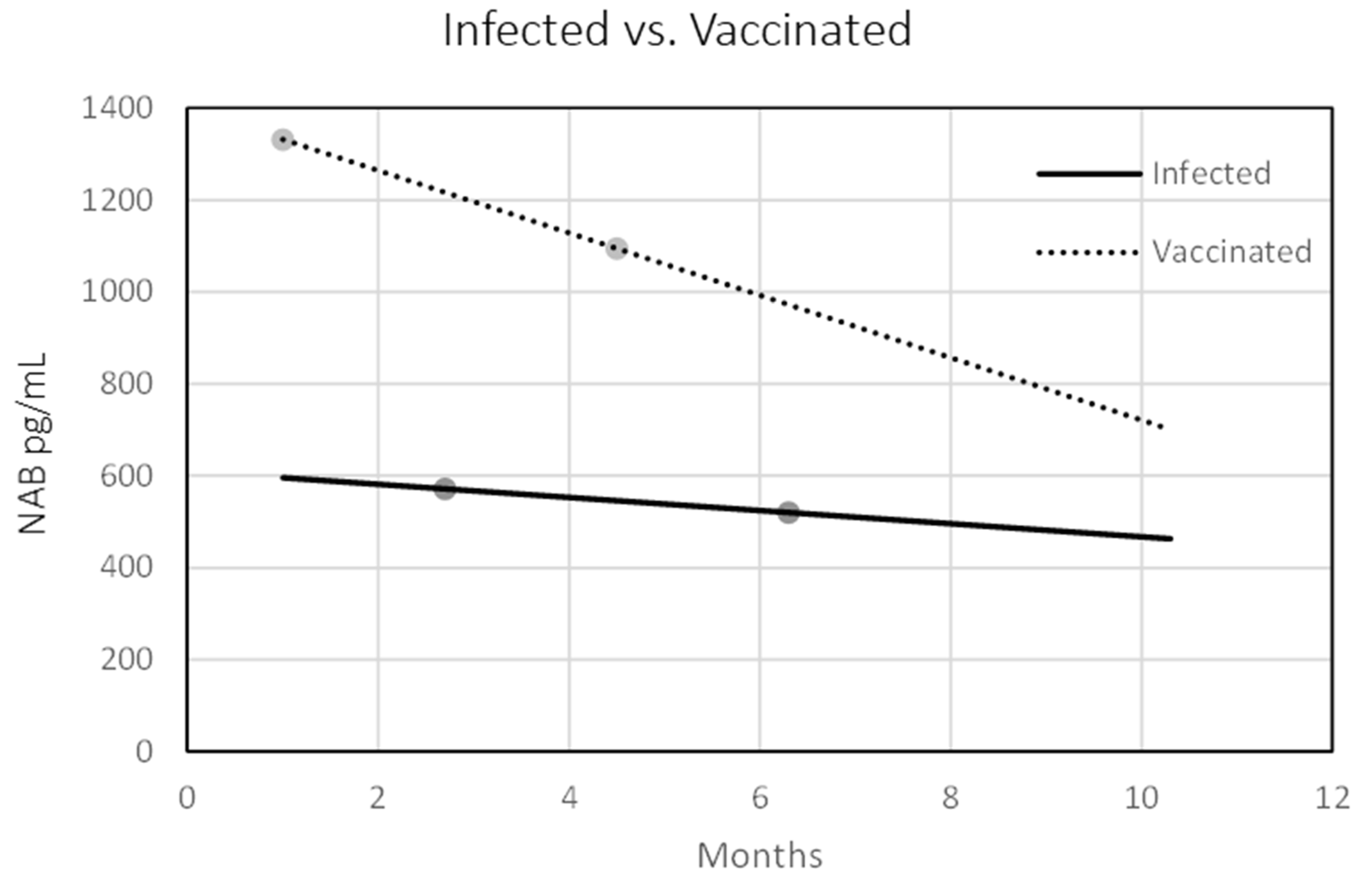
3.1. Κinetics of Anti-RBD, Anti-S1, and Neutralizing Antibodies in Convalescent and Vaccinated Individuals
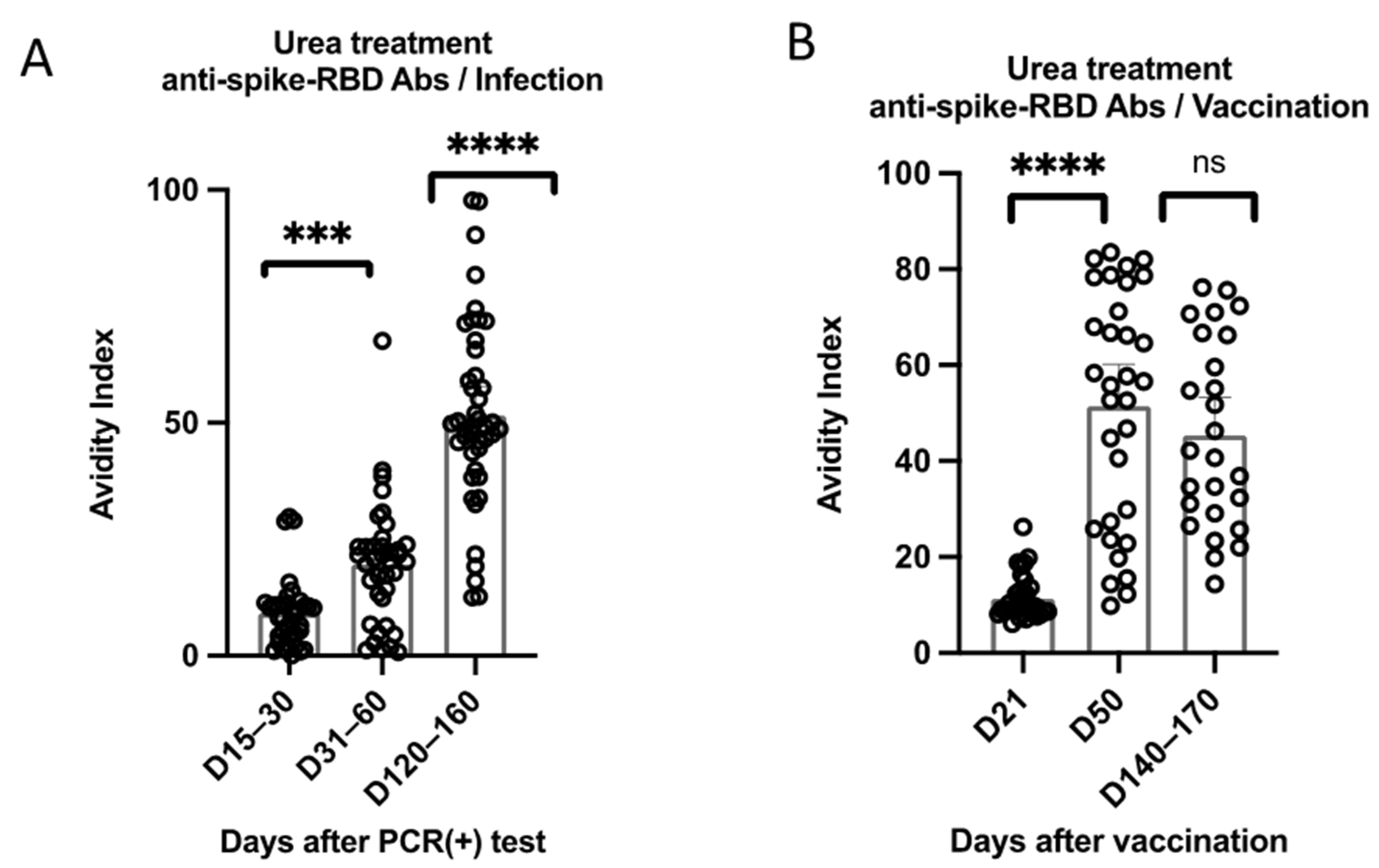
3.2. Kinetics of Anti-RBD Antibody Avidity
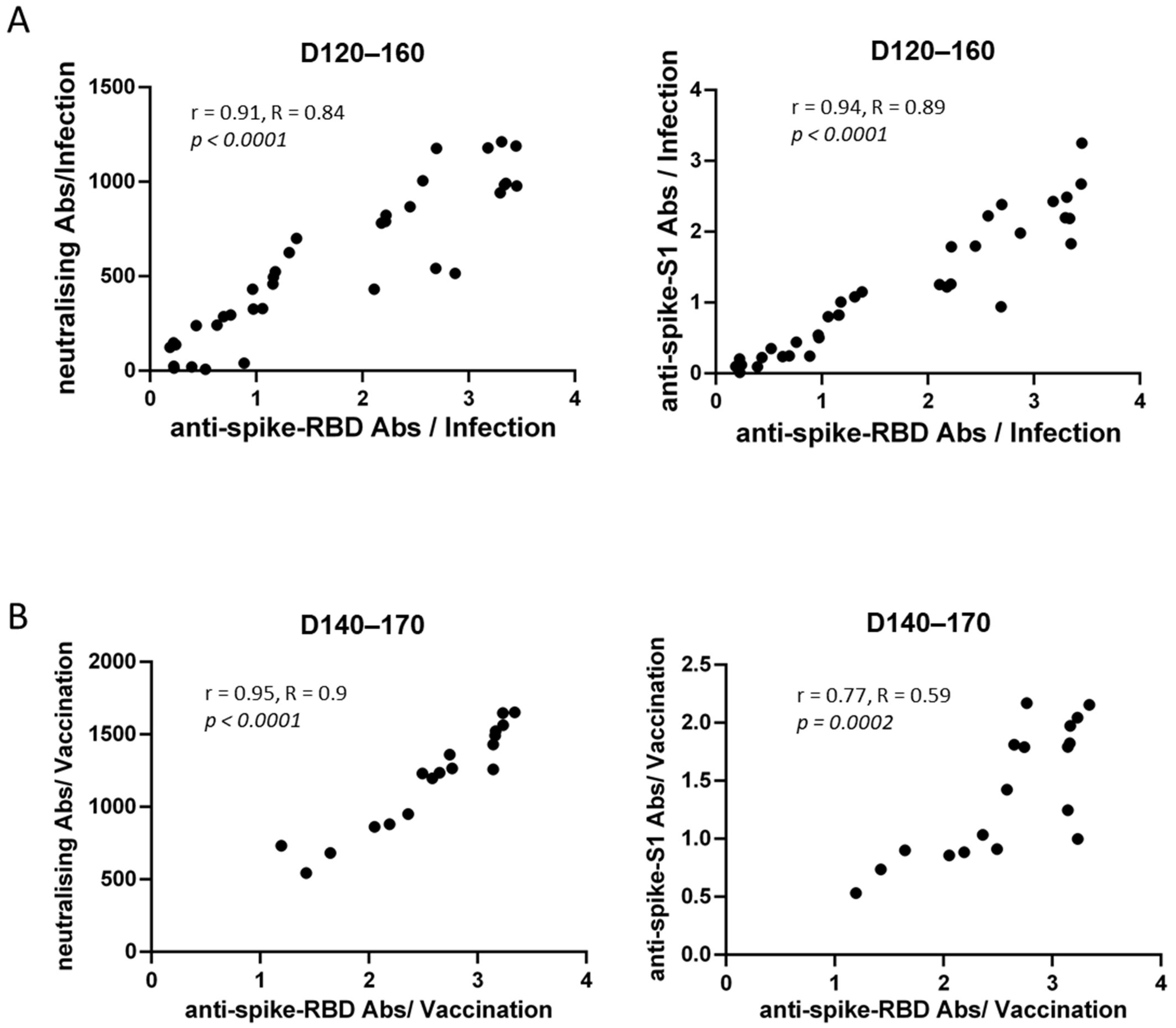
3.3. Correlations of Anti-SARS-CoV-2 Antibody Titers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. MRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Papastamatiou, T.; Routsias, J.G.; Koutsoni, O.; Dotsika, E.; Tsakris, A.; Spoulou, V. Evaluation of Protective Efficacy of Selected Immunodominant B-Cell Epitopes within Virulent Surface Proteins of Streptococcus Pneumoniae. Infect. Immun. 2018, 86, e00673–17. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yu, D.; Wu, X.; Liang, H.; Zhou, Z.; Xie, Y.; Li, T.; Wu, J.; Lu, F.; Feng, L.; et al. Twelve-Month Specific IgG Response to SARS-CoV-2 Receptor-Binding Domain among COVID-19 Convalescent Plasma Donors in Wuhan. Nat. Commun. 2021, 12, 4144. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2022, 10, 64. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Gumeni, S.; Malandrakis, P.; Papanagnou, E.D.; Kastritis, E.; Trougakos, I.P.; Dimopoulos, M.A. Robust Neutralizing Antibody Responses 6 Months Post Vaccination with Bnt162b2: A Prospective Study in 308 Healthy Individuals. Life 2021, 11, 1077. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Vicenti, I.; Basso, M.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Modolo, E.; Dragoni, F.; Parisi, S.G.; Zazzi, M. Faster Decay of Neutralizing Antibodies in Never Infected than Previously Infected Healthcare Workers Three Months after the Second BNT162b2 MRNA COVID-19 Vaccine Dose. Int. J. Infect. Dis. 2021, 112, 40–44. [Google Scholar] [CrossRef]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing Antibody Titers Six Months after Comirnaty Vaccination: Kinetics and Comparison with SARS-CoV-2 Immunoassays. Clin. Chem. Lab. Med. 2022, 60, 456–463. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust Neutralizing Antibodies to SARS-CoV-2 Infection Persist for Months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Muecksch, F.; Wise, H.; Batchelor, B.; Squires, M.; Semple, E.; Richardson, C.; McGuire, J.; Clearly, S.; Furrie, E.; Greig, N.; et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J. Infect. Dis. 2021, 223, 389–398. [Google Scholar] [CrossRef]
- Capetti, A.F.; Borgonovo, F.; Mileto, D.; Gagliardi, G.; Mariani, C.; Lupo, A.; Dedivitiis, G.; Meraviglia, P.; Pellicciotta, M.; Armiento, L.; et al. One-Year Durability of Anti-Spike IgG to SARS-CoV-2: Preliminary Data from the Anticrown Prospective Observational Study One Year Durability of COVID-19 Anti-Spike IgG. J. Infect. 2021, 83, 237–279. [Google Scholar]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 MRNA Vaccines Induce Persistent Human Germinal Centre Responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal Observation and Decline of Neutralizing Antibody Responses in the Three Months Following SARS-CoV-2 Infection in Humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Löfström, E.; Eringfält, A.; Kötz, A.; Wickbom, F.; Tham, J.; Lingman, M.; Nygren, J.M.; Undén, J. Dynamics of IgG-Avidity and Antibody Levels after COVID-19. J. Clin. Virol. 2021, 144, 104986. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising Antibody Titres as Predictors of Protection against SARS-CoV-2 Variants and the Impact of Boosting: A Meta-Analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Zhang, H.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 Antibody Response up to 10 Months after Infection. Cell. Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef]
- Crawford, K.H.D.; Dingens, A.S.; Eguia, R.; Wolf, C.R.; Wilcox, N.; Logue, J.K.; Shuey, K.; Casto, A.M.; Fiala, B.; Wrenn, S.; et al. Dynamics of Neutralizing Antibody Titers in the Months after Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 223, 197–205. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, Y.; Wang, X.; et al. SARS-CoV-2-Specific Antibody and T-Cell Responses 1 Year after Infection in People Recovered from COVID-19: A Longitudinal Cohort Study. Lancet Microbe 2022, 5247, e348–e356. [Google Scholar] [CrossRef]
- Lagousi, T.; Routsias, J.; Spoulou, V. Development of an Enzyme-Linked Immunosorbent Assay (ELISA) for Accurate and Prompt Coronavirus Disease 2019 (COVID-19) Diagnosis Using the Rational Selection of Serological Biomarkers. Diagnostics 2021, 11, 1970. [Google Scholar] [CrossRef]
| Vaccinated | Convalescent | ||
|---|---|---|---|
| n | 36 | 38 | |
| Samples included at each timepoint | D21/D15–30 | 36 | 38 |
| D50/D31–60 | 32 | 38 | |
| D140–170/D120–160 | 26 | 34 | |
| Age | Median (in years) | 37 | 39 |
| Range (in years) | 24–52 | 22–53 | |
| Gender | Female (n, %) | 21 (58.3%) | 22 (57.9%) |
| Male (n, %) | 15 (41.7%) | 16 (42.1%) | |
| Body Mass Index (BMI) | Mean (SD) | 25.1 (4.4) | 25.7 (4.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagousi, T.; Routsias, J.; Mavrouli, M.; Papadatou, I.; Geropeppa, M.; Spoulou, V. Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection. Vaccines 2022, 10, 1210. https://doi.org/10.3390/vaccines10081210
Lagousi T, Routsias J, Mavrouli M, Papadatou I, Geropeppa M, Spoulou V. Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection. Vaccines. 2022; 10(8):1210. https://doi.org/10.3390/vaccines10081210
Chicago/Turabian StyleLagousi, Theano, John Routsias, Maria Mavrouli, Ioanna Papadatou, Maria Geropeppa, and Vana Spoulou. 2022. "Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection" Vaccines 10, no. 8: 1210. https://doi.org/10.3390/vaccines10081210
APA StyleLagousi, T., Routsias, J., Mavrouli, M., Papadatou, I., Geropeppa, M., & Spoulou, V. (2022). Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection. Vaccines, 10(8), 1210. https://doi.org/10.3390/vaccines10081210





