Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Models
2.3. Vaccine Preparation
2.4. Vaccine Immunogenicity Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Microneutralization Assay
2.7. Cytokine Analysis Assay
2.8. Vaccine Safety Evaluation
2.9. Pathological Examination
2.10. Statistical Analysis
3. Results
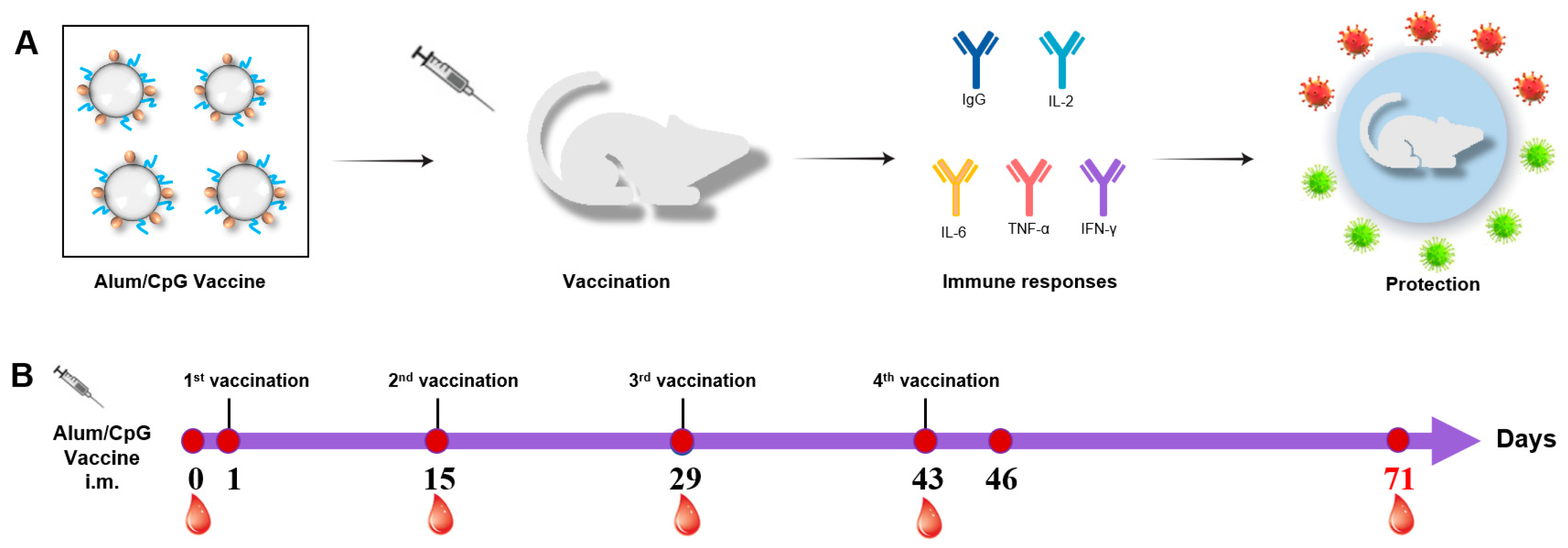
3.1. Procedures for Immunization and Sample Collection
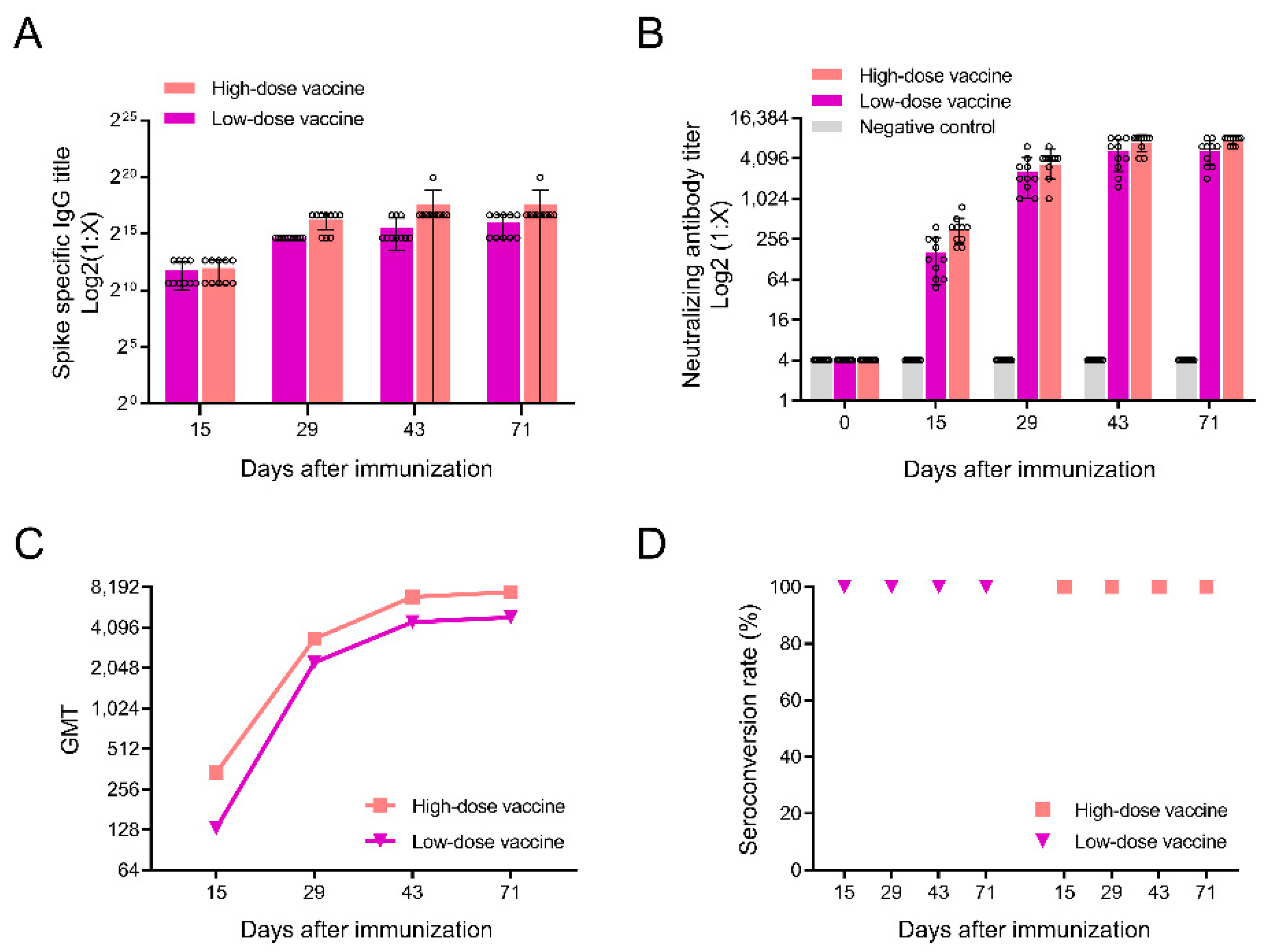
3.2. Detection of Antibodies after Immunization in Rats
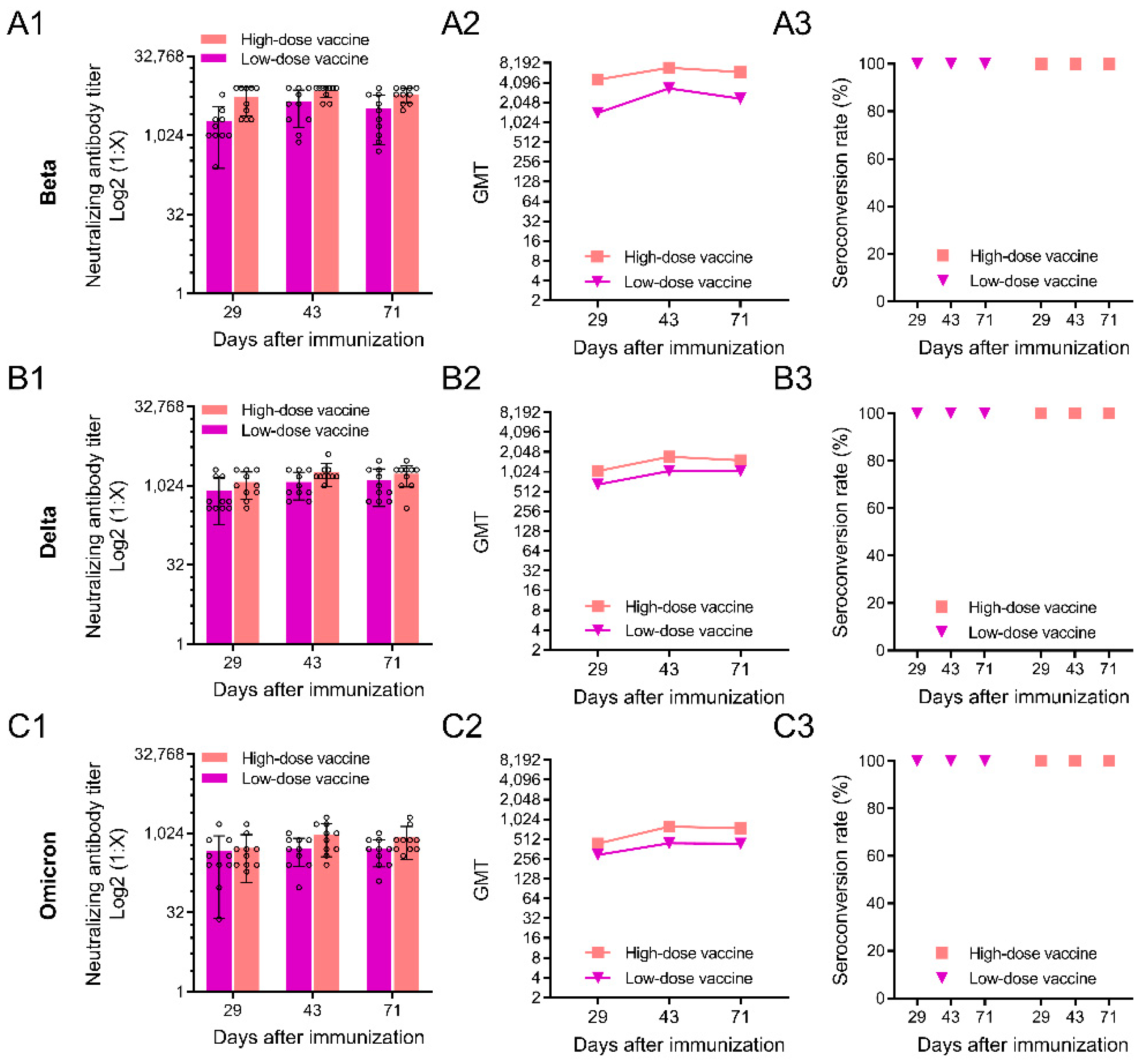
3.3. Neutralizing Antibody Responses against SARS-CoV-2 Variants
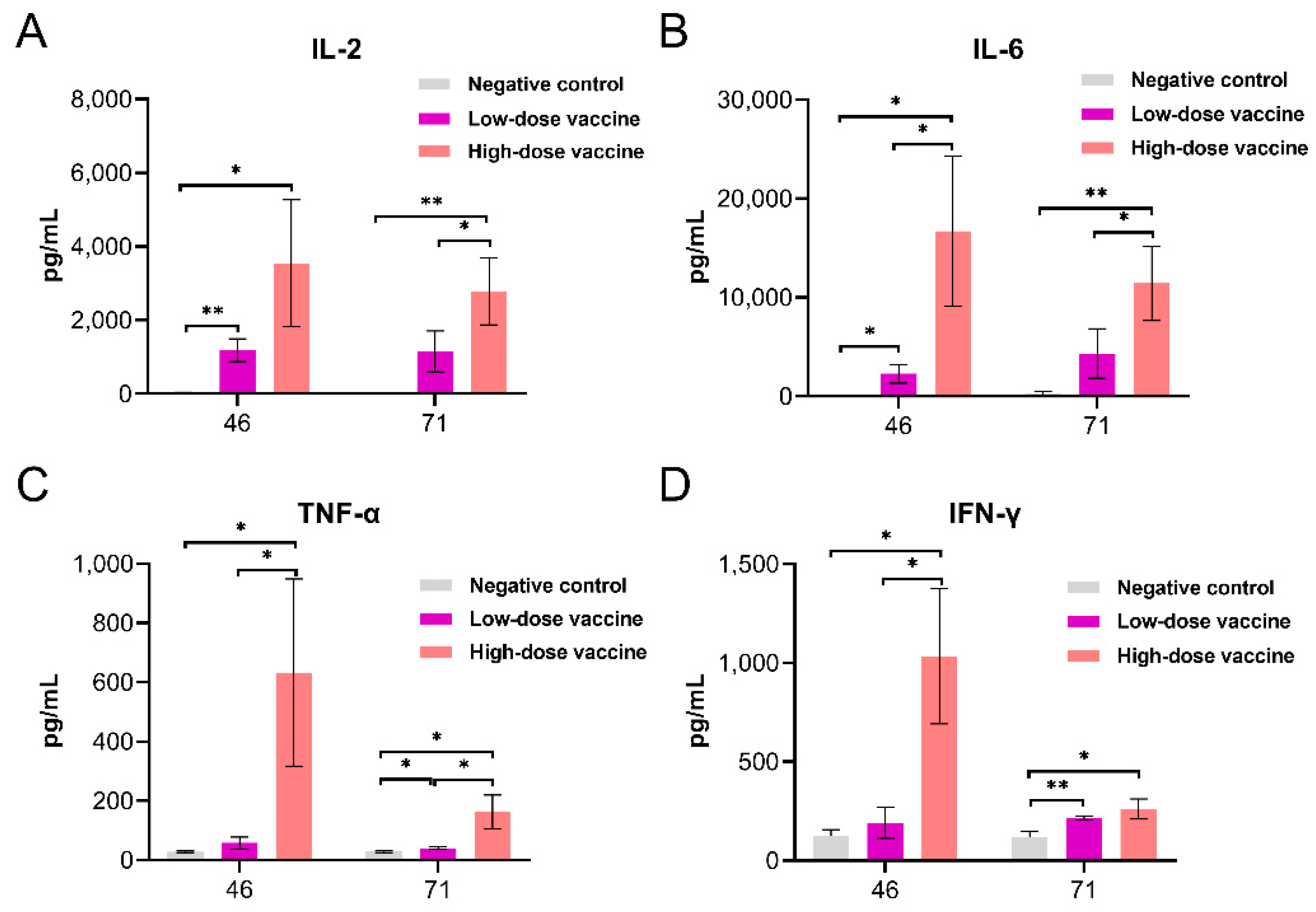
3.4. Cytokines
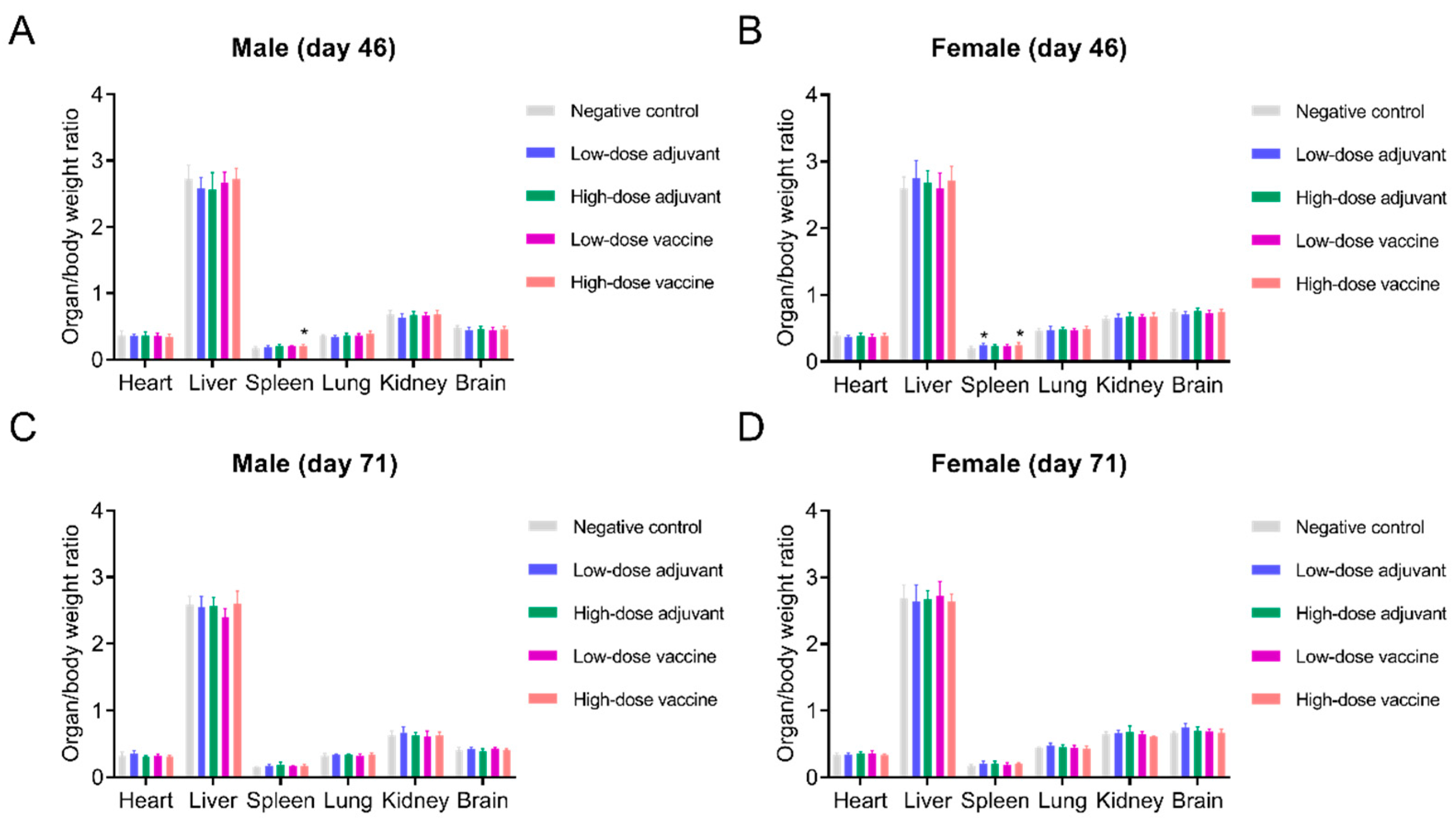
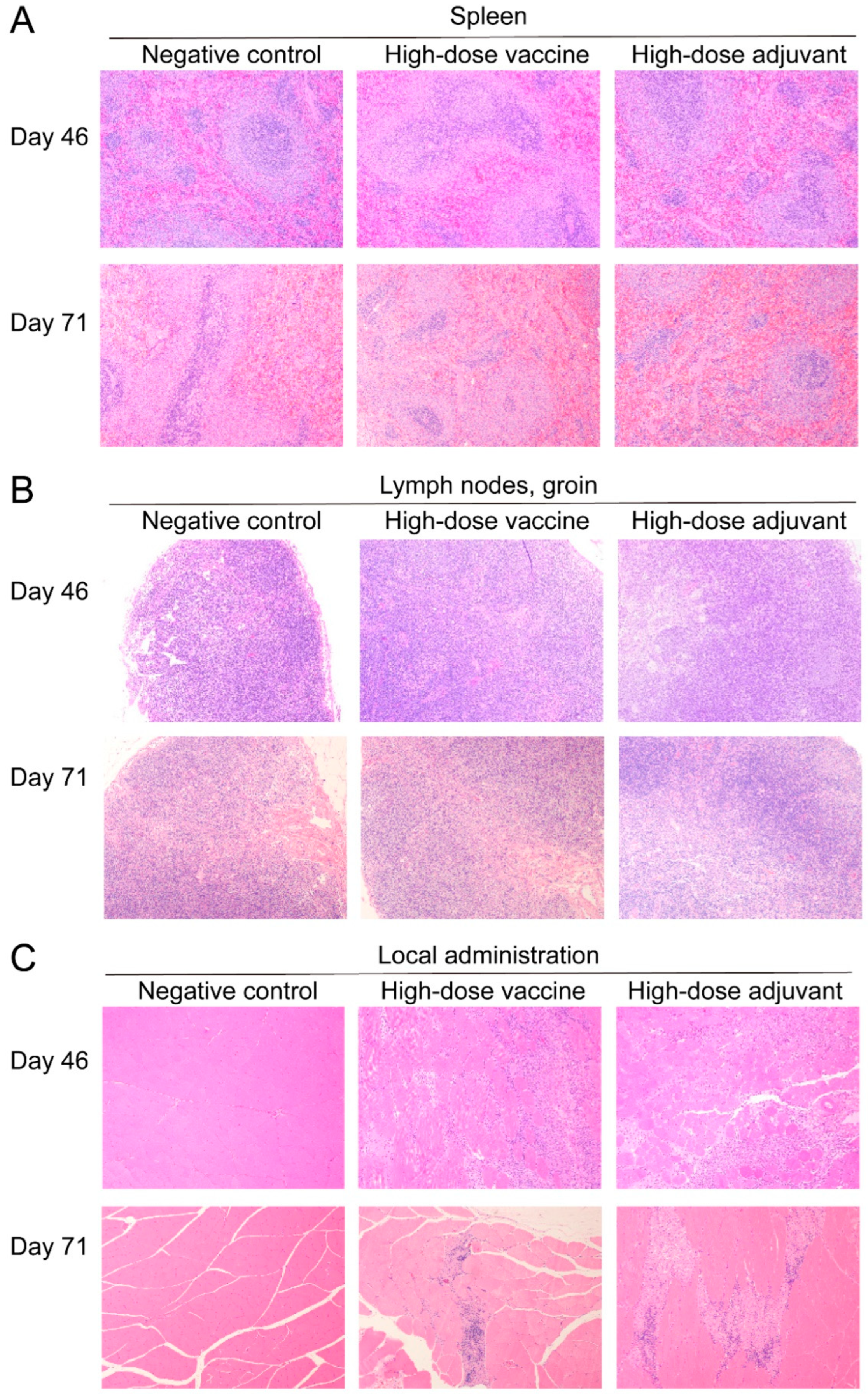
3.5. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Adapa, S.; Aeddula, N.R.; Roy, S.; Nandikanti, D.; Vupadhyayula, P.M.; Naramala, S.; Gayam, V.; Muppidi, V.; Konala, V.M. Medical Management of COVID-19: Evidence and Experience. J. Clin. Med. Res. 2020, 12, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. The success of SARS-CoV-2 vaccines and challenges ahead. Cell Host Microbe 2021, 29, 1111–1123. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Wrobel, A.G.; Benton, D.J.; Roustan, C.; Borg, A.; Hussain, S.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Evolution of the SARS-CoV-2 spike protein in the human host. Nat. Commun. 2022, 13, 1178. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Xiao, T.; Peng, H.; Sterling, S.M.; Walsh, R.M., Jr.; Rawson, S.; Rits-Volloch, S.; Chen, B. Distinct conformational states of SARS-CoV-2 spike protein. Science 2020, 369, 1586–1592. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Scheepers, C.; Everatt, J.; Amoako, D.G.; Tegally, H.; Wibmer, C.K.; Mnguni, A.; Ismail, A.; Mahlangu, B.; Lambson, B.E.; Martin, D.P.; et al. Emergence and phenotypic characterization of the global SARS-CoV-2 C.1.2 lineage. Nat. Commun. 2022, 13, 1976. [Google Scholar] [CrossRef]
- Ye, G.; Liu, B.; Li, F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat. Commun. 2022, 13, 1214. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2523. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e1014. [Google Scholar] [CrossRef]
- Olusanya, O.A.; Bednarczyk, R.A.; Davis, R.L.; Shaban-Nejad, A. Addressing Parental Vaccine Hesitancy and Other Barriers to Childhood/Adolescent Vaccination Uptake During the Coronavirus (COVID-19) Pandemic. Front. Immunol. 2021, 12, 663074. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Harris, R.J.; Hall, J.A.; Zaidi, A.; Andrews, N.J.; Dunbar, J.K.; Dabrera, G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N. Engl. J. Med. 2021, 385, 759–760. [Google Scholar] [CrossRef]
- Bian, L.; Gao, F.; Zhang, J.; He, Q.; Mao, Q.; Xu, M.; Liang, Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 365–373. [Google Scholar] [CrossRef]
- Burki, T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 2022, 10, e17. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Ho, T.C.; Chen, Y.A.; Chan, H.P.; Chang, C.C.; Chuang, K.P.; Lee, C.H.; Yuan, C.H.; Tyan, Y.C.; Yang, M.H. The Effects of Heterologous Immunization with Prime-Boost COVID-19 Vaccination against SARS-CoV-2. Vaccines 2021, 9, 1163. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Wu, J.N.; Huang, R.D.; Xie, F.Q.; Li, J.R.; Zheng, K.C.; Zhang, D.J. Comparison of Safety of Different Vaccine Boosters Following Two-Dose Inactivated Vaccines: A Parallel Controlled Prospective Study. Vaccines 2022, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.K. The guiding principles for populational COVID-19 vaccine selection: A normative analysis through comparison of the strategies in Hong Kong and Singapore. J. Glob. Health 2022, 12, 3004. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Mirotti, L.; Alberca Custódio, R.W.; Gomes, E.; Rammauro, F.; de Araujo, E.F.; Garcia Calich, V.L.; Russo, M. CpG-ODN Shapes Alum Adjuvant Activity Signaling via MyD88 and IL-10. Front. Immunol. 2017, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Beran, J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin. Biol. Ther. 2008, 8, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Shu-zhen, J. Effect of BCG-CpG-DNA as An Immunoadjuvant of Recombinant HBsAg. Chin. J. Biol. 2007, 20, 356–361. [Google Scholar]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef]
- Krieg, A.M.; Davis, H.L. Enhancing vaccines with immune stimulatory CpG DNA. Curr. Opin. Mol. Ther. 2001, 3, 15–24. [Google Scholar]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e719. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Dashdorj, N.J.; Wirz, O.F.; Röltgen, K.; Haraguchi, E.; Buzzanco, A.S., 3rd; Sibai, M.; Wang, H.; Miller, J.A.; Solis, D.; Sahoo, M.K.; et al. Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe 2021, 29, 1738–1743.e1734. [Google Scholar] [CrossRef]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castaño, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e1015. [Google Scholar] [CrossRef]
- McLean, G.; Kamil, J.; Lee, B.; Moore, P.; Schulz, T.F.; Muik, A.; Sahin, U.; Türeci, Ö.; Pather, S. The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. mBio 2022, 13, e0297921. [Google Scholar] [CrossRef]
- Alkhatib, M.; Salpini, R.; Carioti, L.; Ambrosio, F.A.; D’Anna, S.; Duca, L.; Costa, G.; Bellocchi, M.C.; Piermatteo, L.; Artese, A.; et al. Update on SARS-CoV-2 Omicron Variant of Concern and Its Peculiar Mutational Profile. Microbiol. Spectr. 2022, 10, e0273221. [Google Scholar] [CrossRef]
- Yan, X.; Chen, G.; Jin, Z.; Zhang, Z.; Zhang, B.; He, J.; Yin, S.; Huang, J.; Fan, M.; Li, Z.; et al. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J. Med. Virol. 2022, 94, 380–383. [Google Scholar] [CrossRef]
- Moradi, G.; Mohamadi Bolbanabad, A.; Ahmadi, S.; Aghaei, A.; Bahrami, F.; Veysi, A.; Nasiri Kalmarzi, R.; Shokri, A.; Ghaderi, E.; Mohsenpour, B.; et al. Persistence assessment of SARS-CoV-2-specific IgG antibody in recovered COVID-19 individuals and its association with clinical symptoms and disease severity: A prospective longitudinal cohort study. Int. Immunopharmacol. 2021, 98, 107893. [Google Scholar] [CrossRef]
- Carrillo, J.; Izquierdo-Useros, N.; Ávila-Nieto, C.; Pradenas, E.; Clotet, B.; Blanco, J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2021, 538, 187–191. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zheng, X.; Sheng, W.; Liang, H.; Zhao, Y.; Zhu, X.; Yang, R.; Zhang, Y.; Dong, X.; Li, W.; et al. Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants. Vaccines 2022, 10, 1208. https://doi.org/10.3390/vaccines10081208
Zhang Y, Zheng X, Sheng W, Liang H, Zhao Y, Zhu X, Yang R, Zhang Y, Dong X, Li W, et al. Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants. Vaccines. 2022; 10(8):1208. https://doi.org/10.3390/vaccines10081208
Chicago/Turabian StyleZhang, Yuntao, Xiaotong Zheng, Wang Sheng, Hongyang Liang, Yuxiu Zhao, Xiujuan Zhu, Rong Yang, Yadan Zhang, Xiaofei Dong, Weidong Li, and et al. 2022. "Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants" Vaccines 10, no. 8: 1208. https://doi.org/10.3390/vaccines10081208
APA StyleZhang, Y., Zheng, X., Sheng, W., Liang, H., Zhao, Y., Zhu, X., Yang, R., Zhang, Y., Dong, X., Li, W., Pei, F., Ding, L., Chang, Z., Deng, L., Yuan, G., Yang, Z., Zhu, D., Yang, X., & Wang, H. (2022). Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants. Vaccines, 10(8), 1208. https://doi.org/10.3390/vaccines10081208





