Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Study Population
- Inclusion Criteria
- Subjects aged 40 and over
- Having received two doses of one of the following vaccines mRNA-1273 or Spikevax (Moderna), BNT162B2 or Comirnaty (Pfizer-BioNTech), Gam-COVID-Vac or Sputnik V (Gamaleya Research Institute), ChAdOx1-S or Vaxzevria (Astrazeneca), BIBP (Sinopharm) and Coronavac (Sinovac)
- Vaccinated at one of the vaccination centers of the governorates of Tunis, Nabeul, Bizerte, Sousse, Monastir, and Mahdia
- Having given informed consent
- Exclusion Criteria
- Subjects having had symptomatic COVID-19 before or after vaccination.
- Pregnant women
- Immunocompromised patients or under immunosuppressive treatments.
2.3. Sampling
2.4. Peripheral Anti-N and Anti-S Antibody Measurement
2.5. Cellular Immunity Analysis
2.6. Statistical Analyses
3. Results
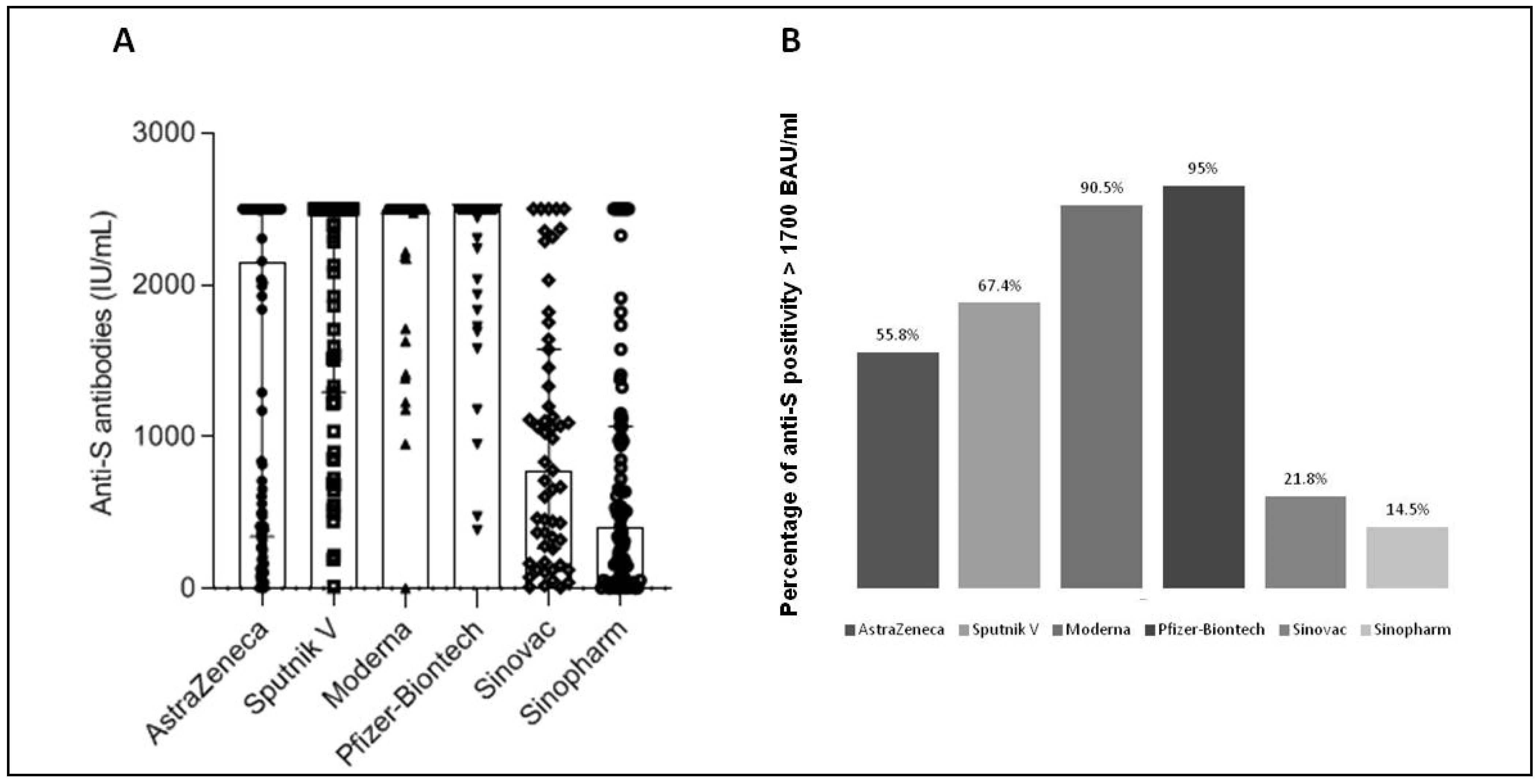
3.1. Antibody Development after SARS-CoV-2 Vaccination
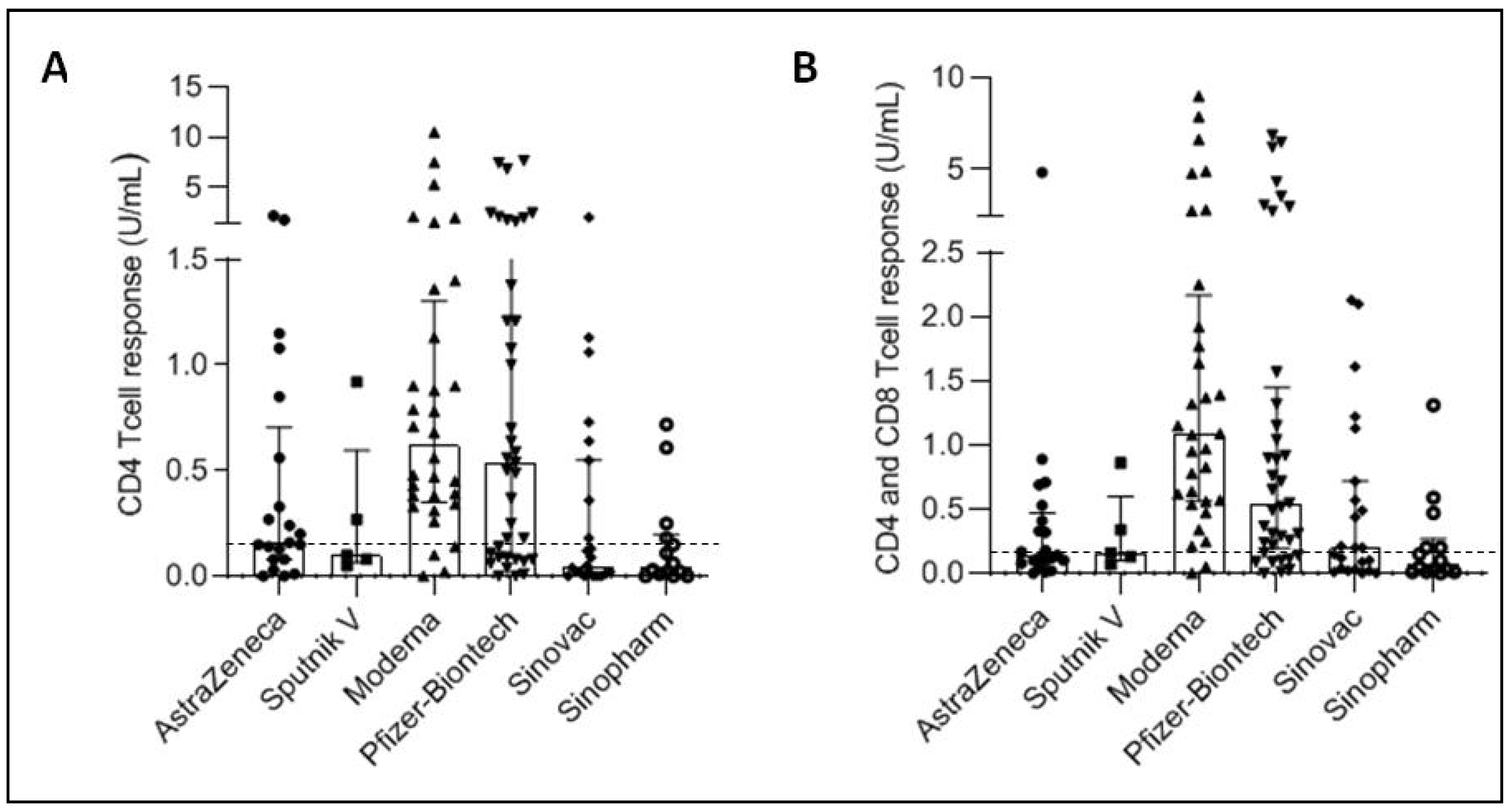
3.2. Cellular Immune Response Development after SARS-CoV-2 Vaccination
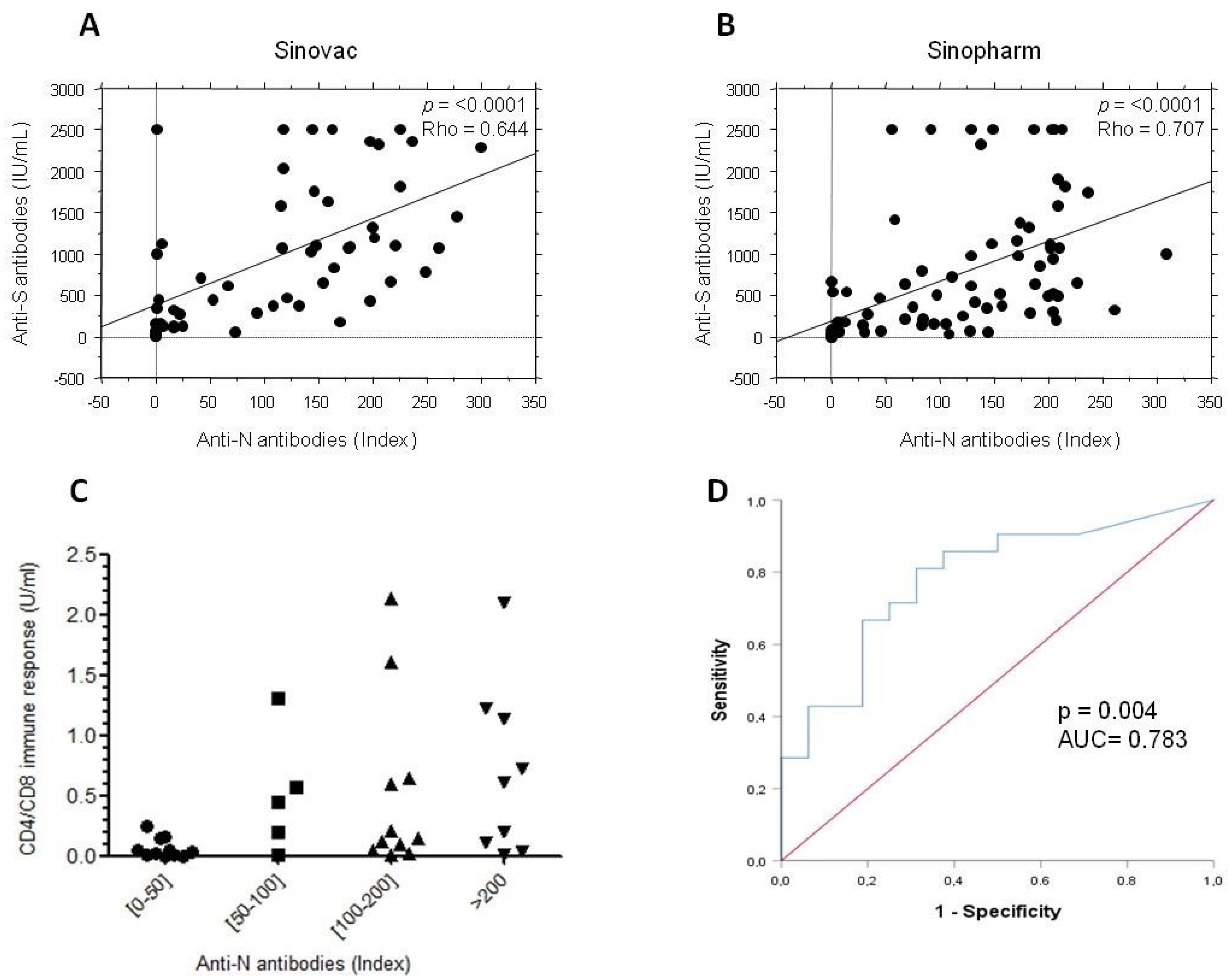
3.3. Correlation between Humoral and Cellular Responses Post-SARS-CoV-2 Vaccination
3.4. Association of the Post-Vaccination Immune Response with Different Clinical Parameters
3.5. Anti-N Antibodies in Subjects Who Received Inactivated Vaccines
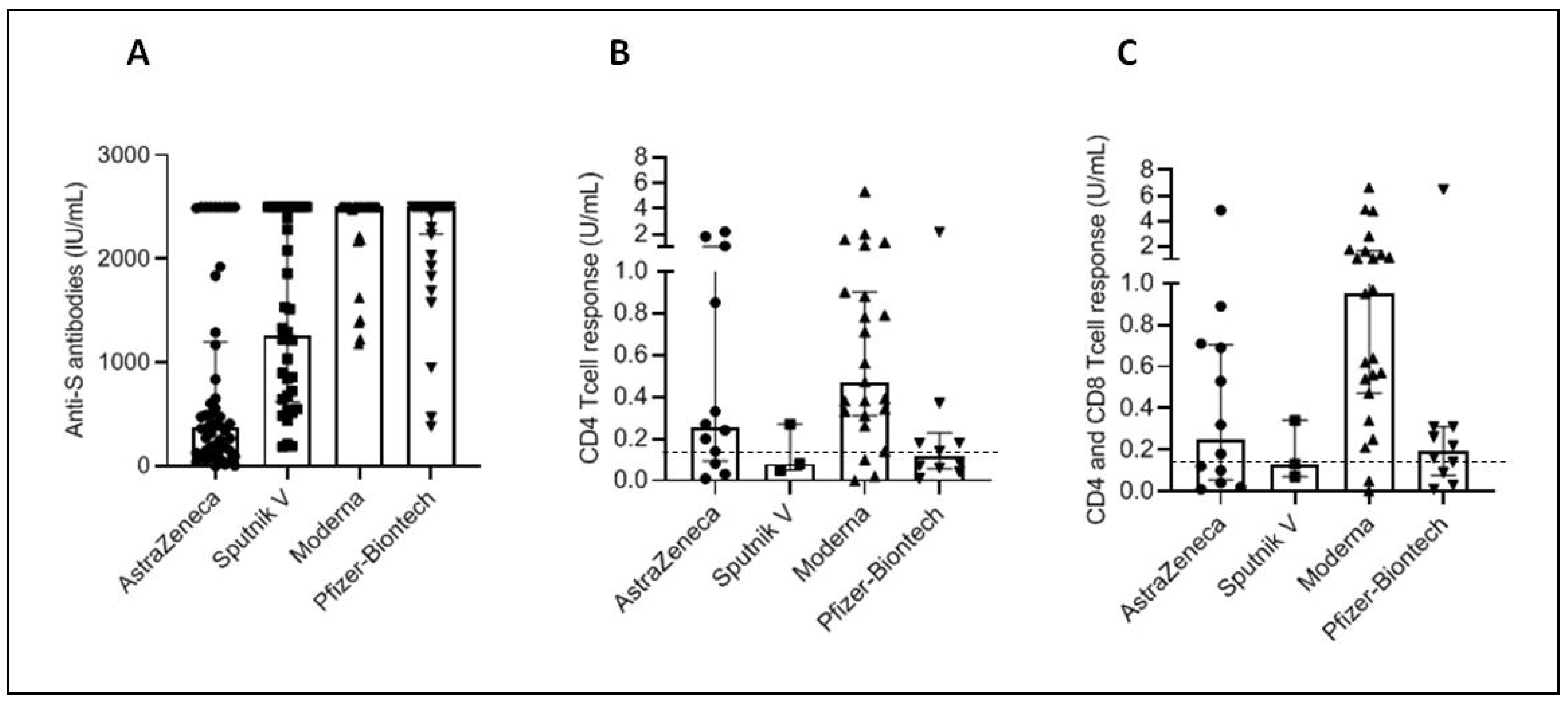
3.6. Antibody and Cellular Immunity in People with Negative Anti-N Antibodies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int (accessed on 12 January 2022).
- Available online: http://www.santetunisie.rns.tn/images/plan-riposte-covid19.pdf. (accessed on 15 July 2021).
- Safer, M.; Letaief, H.; Hechaichi, A.; Harizi, C.; Dhaouadi, S.; Bouabid, L.; Darouiche, S.; Gharbi, D.; Elmili, N.; Salah, H.B.; et al. Identification of transmission chains and clusters associated with COVID-19 in Tunisia. BMC. Infect. Dis. 2021, 21, 453. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody titers and protection against a SARS-CoV-2 infection. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef]
- Lim, W.W.; Mak, L.; Leung, G.M.; Cowling, B.J.; Peiris, M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021, 2, e423. [Google Scholar] [CrossRef]
- Seija, M.; Rammauro, F.; Santiago, J.; Orihuela, N.; Zulberti, C.; Machado, D.; Recalde, C.; Noboa, J.; Frantchez, V.; Astesiano, R.; et al. Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin. Kidney J. 2021, 15, 527–533. [Google Scholar] [CrossRef]
- Haller, M.C.; Kaiser, R.A.; Langthaler, S.; Brandstetter, C.; Apfalter, P.; Kerschner, H.; Cejka, D. Comparison of mRNA-1273 and BNT162b2 SARS-CoV-2 mRNA Vaccine Immunogenicity in Kidney Transplant Recipients. Transpl. Int. 2022, 35, 10026. [Google Scholar] [CrossRef]
- Petrović, V.; Vuković, V.; Patić, A.; Marković, M.; Ristić, M. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS-CoV-2 infection-A comparative study from Novi Sad, Serbia. PLoS ONE 2022, 17, e0263468. [Google Scholar] [CrossRef]
- Ozakbas, S.; Baba, C.; Dogan, Y.; Cevik, S.; Ozcelik, S.; Kaya, E. Comparison of SARS-CoV-2 antibody response after two doses of mRNA and inactivated vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult. Scler. Relat. Disord. 2022, 58, 103486. [Google Scholar] [CrossRef]
- Ionita, C.; Marcelli, D.; Nita, C.; Anton, C.; Berca, S.; Vacar, S.; Schiller, O.; Gheorghiu, C.; Barth, C. Comparison of antibody response to two different mRNA COVID-19 vaccines in patients on hemodialysis. J. Nephrol. 2022, 35, 143–151. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, S.-H.; Kim, B.; Kim, J.-Y.; Ji, Y.-S.; Kim, S.-H.; Kim, C.-K.; Kim, T.; Choo, E.-J.; Moon, J.-E.; et al. Serum Antibody Response Comparison and Adverse Reaction Analysis in Healthcare Workers Vaccinated with the BNT162b2 or ChAdOx1 COVID-19 Vaccine. Vaccines 2021, 9, 1379. [Google Scholar] [CrossRef]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.M.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Rahim, H.F.A.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naive and previously infected individuals. J. Travel. Med. 2021, 28, taab190. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.-D.; Jo, D.H.; Choe, K.-W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.P.; Cohen, C.A.; Cheng, S.M.S.; Chen, C.; Kwok, K.-O.; You, K.; Chan, T.-O.; Bull, M.; Ling, K.C.; Dai, Z.; et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology 2021, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Vályi-Nagy, I.; Matula, Z.; Gönczi, M.; Tasnády, S.; Bekő, G.; Réti, M.; Ajzner, E.; Uher, F. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. Geroscience 2021, 43, 2321–2331. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef]
- Kaiser, R.A.; Haller, M.C.; Apfalter, P.; Kerschner, H.; Cejka, D. Comparison of BNT162b2 (Pfizer-BioNtech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021, 100, 697–698. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Gam-COVID-Vac Vaccine Trial Group Safety and efficacy of a rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Dimeglio, C.; Migueres, M.; Mansuy, J.-M.; Saivin, S.; Miedougé, M.; Chapuy-Regaud, S.; Izopet, J. Antibody titers and breakthrough infections with Omicron SARS-CoV-2. J. Infect. 2022, 84, e13–e15. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, M.; Lee, S.; Kim, G.; Choe, P.G.; Park, W.B.; Kim, N.J.; Lee, C.-H.; Kim, I.S.; Jung, K.; et al. Longitudinal analysis of human memory T-cell response according to the severity of illness up to 8 months after severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021, 224, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Laing, E.D.; DaMata, J.P.; Pohida, K.; Tso, M.S.; Samuels, E.C.; Epsi, N.J.; Dorjbal, B.; Lake, C.; Richard, S.A.; et al. Durability of SARS-CoV-2-Specific T-Cell Responses at 12 Months Postinfection. J. Infect. Dis. 2021, 224, 2010–2019. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4 + and CD8 + T cell reactivity in infected or vaccinated individuals. Cell. Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Abdul Quadeer, A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- Goletti, D.; Petrone, L.; Manissero, D.; Bertoletti, A.; Rao, S.; Ndunda, N.; Sette, A.; Nikolayevskyy, V. The potential clinical utility of measuring SARS-CoV-2-specific T-cell responses. Clin. Microbiol. Infect. 2021, 27, 1784–1789. [Google Scholar] [CrossRef]
- Krüttgen, A.; Klingel, H.; Haase, G.; Haefner, H.; Imöhl, M.; Kleines, M. Evaluation of the QuantiFERON SARS-CoV-2 interferon-ɣ release assay in mRNA-1273 vaccinated health care workers. J. Virol. Methods 2021, 298, 114295. [Google Scholar] [CrossRef]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Altamirano, J.; Maldonado, Y.A.; Bonilla, H.F.; Jacobson, K.B.; Parsonnet, J.; Andrews, J.R.; Shi, R.-Z.; Boyd, S.; et al. Long Term Accuracy of SARS-CoV-2 Interferon-γ Release Assay and its Application in Household Investigation. Clin. Infect. Dis. 2022, 25, ciac045. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.Z.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488. [Google Scholar] [CrossRef]
- Nakagama, Y.; Nitahara, Y.; Kaku, N.; Tshibangu-Kabamba, E.; Kido, Y. A Dual-Antigen SARS-CoV-2 Serological Assay Reflects Antibody Avidity. J. Clin. Microbiol. 2022, 60, e0226221. [Google Scholar] [CrossRef]

| N | Median Age (IQR) | Sex M/F | Diabetes N (%) | Hypertension N (%) | Obesity N (%) | Cardiovascular Disease N (%) | Asthma N (%) | |
|---|---|---|---|---|---|---|---|---|
| Astrazeneca | 95 | 50 (44–57) | 43/52 | 10 (10.5) | 15 (15.8) | 6 (6.3) | 3 (3.2) | 6 (6.3) |
| Sputnik V | 86 | 55 (50–59) | 35/51 | 22 (26.5) | 24 (27.9) | 4 (4.7) | 5 (5.8) | 4 (4.7) |
| Moderna | 63 | 43 (41–48) | 26/37 | 6 (9.5) | 4 (6.3) | 4 (6.3) | 0 (0.0) | 1 (1.6) |
| Pfizer-BioNTech | 119 | 56 (51–63) | 55/64 | 31 (26.1) | 28 (23.5) | 15 (12.6) | 9 (7.6) | 4 (3.4) |
| Sinovac | 55 | 55 (49–60) | 26/29 | 10 (18.2) | 10 (18.2) | 5 (9.1) | 2 (3.6) | 4 (7.3) |
| Sinopharm | 83 | 49 (48–51) | 39/44 | 14 (16.9) | 12 (14.5) | 9 (10.8) | 4 (4.8) | 6 (7.2) |
| All vaccines | 501 | 51 (47–58) | 224/277 | 93 (18.6) | 93 (18.6) | 43 (8.6) | 23 (4.6) | 25 (5) |
| N | Anti-S Antibodies Median Level [IQR] | CD4 Response Median Level [IQR] | CD4 + CD8 Response Median Level [IQR] | |
|---|---|---|---|---|
| Astrazeneca | 95 | 2157.00 [340.9–2500.00] | 0.16 [0.08–0.70] | 0.14 [0.09–0.47] |
| Sputnik V | 86 | 2500.00 [1294.50–2500.00] | 0.10 [0.065–0.595] | 0.16 [0.10–0.60] |
| Moderna | 63 | 2500.00 [2500.00–2500.00] | 0.62 [0.35–1.30] | 1.08 [0.56–2.16] |
| Pfizer | 119 | 2500.00 [2500.00–2500.00] | 0.54 [0.09–1.51] | 0.55 [0.19–1.44] |
| Sinovac | 55 | 777.60 [261.00–1575.00] | 0.05 [0.01–0.55] | 0.20 [0.03–0.72] |
| Sinopharm | 83 | 409.50 [60.88–1066.00] | 0.045 [0.0075–0.1975] | 0.075 [0.010–0.267] |
| p value | <0.0001 | <0.0001 | <0.0001 |
| Anti-S Antibodies % of Positivity (>15 IU/mL) | CD4 Response % of Positivity (>0.15 IU/mL) | CD4 + CD8 Response % of Positivity (>0.15 IU/mL) | |
|---|---|---|---|
| Astrazeneca | 97.9 | 47.6 | 38.1 |
| Sputnik V | 98.8 | 40.0 | 40.0 |
| Moderna | 100 | 87.5 | 93.8 |
| Pfizer | 100 | 62.2 | 75.7 |
| Sinovac | 96.4 | 30.4 | 52.2 |
| Sinopharm | 85.5 | 21.4 | 35.7 |
| Anti-S Antibodies | CD4 Response | CD4 + CD8 Response | |
|---|---|---|---|
| Astrazeneca vs.Sputnik V | 0.003 | 0.431 | 0.056 |
| Moderna vs.Pfizer | 0.497 | 0.828 | 0.275 |
| Sinovac vs.Sinopharm | 0.054 | 0.828 | 0.275 |
| RNA vaccines vs.Adenovirus vaccines | <0.0001 | 0.002 | <0.0001 |
| RNA vaccines vs.Inactivated vaccines | <0.0001 | <0.0001 | <0.0001 |
| Adenovirus vaccines vs.Inactivated vaccines | <0.0001 | 0.206 | 0.838 |
| Anti-S vs. CD4 Response | Anti-S vs. CD4 + CD8 Response | |
|---|---|---|
| Astrazeneca | p = 0.135 | p = 0.161 |
| Sputnik V | p = 0.554 | p = 0.605 |
| Moderna | p = 0.605 | p = 0.605 |
| Pfizer | p = 0.521 | p = 0.536 |
| Sinovac | p = 0.006 r = 0.552 | p = 0.017 r = 0.449 |
| Sinopharm | p = 0.015 r = 0.631 | p = 0.013 r = 0.642 |
| Sex (M/F) | Diabetes (Yes/No) | Hypertension (Yes/No) | Obesity (Yes/No) | Cardiovascular Disease (Yes/No) | Asthma (Yes/No) | |
|---|---|---|---|---|---|---|
| Anti-S Antibodies UI/mL | 1624/1771 (p = 0.096) | 1712/1704 (p = 0.943) | 1709/1705 (p = 0.969) | 1872/1690 (p = 0.219) | 1644/1709 (p = 0.776) | 1965/1692 (p = 0.136) |
| CD4 T Cell Response UI/mL | 0.753/0.903 (p = 0.598) | 0.764/0.849 (p = 0.822) | 1.619/0.734 (p = 0.179) | 0.500/0.840 (p = 0.618) | 1.943/1.782 (p = 0.367) | 0.858/0.833 (p = 0.947) |
| CD4 + CD8 T Cell Response UI/mL | 0.948/1.131 (p = 0.540) | 0.746/1.108 (p = 0.275) | 1.372/1.006 (p = 0.451) | 0.450/1.057 (p = 0.390) | 1.955/1.004 (p = 0.366) | 1.311/1.033 (p = 0.689) |
| Anti-S Antibodies | CD4 Response | CD4/CD8 Response | |
|---|---|---|---|
| Astrazeneca vs. Sputnik V | 0.0001 | 0.311 | 0.663 |
| Moderna vs. Pfizer | 0.595 | 0.011 | 0.006 |
| RNA vaccines vs. Adenovirus vaccines | <0.0001 | 0.379 | 0.049 |
| Positive Anti-N Antibodies | Negative Anti-N Antibodies | |||||
|---|---|---|---|---|---|---|
| Anti-S Antibodies Median Level [IQR] | CD4 Response Median Level [IQR] | CD4 and CD8 Response Median Level [IQR] | Anti-S Antibodies Median Level [IQR] | CD4 Response Median Level [IQR] | CD4 and CD8 Response Median Level [IQR] | |
| Astrazeneca | 2500.00 [2500.00–2500.00] | 0.15 [0.04–0.36] | 0.12 [0.09–0.25] | 376.45 [144.975–1201.50] | 0.255 [0.095–1.022] | 0.25 [0.055–0.705] |
| Sputnik V | 2500.00 [2500.00–2500.00] | 0.51 [0.10–0.62] | 0.51 [0.16–0.61] | 1261.50 [622.15–2500.00] | 0.08 [0.05–0.29] | 0.13 [0.07–0.34] |
| Moderna | 2500.00 [2500.00–2500.00] | 0.90 [0.456–4.81] | 1.92 [1.07–5.33] | 2500.00 [2488.00–2500.00] | 0.47 [0.31–0.90] | 0.95 [0.47–1.64] |
| Pfizer | 2500.00 [2500.00–2500.00] | 0.70 [0.25–1.82] | 0.89 [0.37–2.74] | 2500.00 [2239.00–2500.00] | 0.115 [0.055–0.227] | 0.19 [0.075–0.31] |
| Sinovac | 1026.00 [367.95–1696.50] | 0.09 [0.02–0.64] | 0.21 [0.04–1.13] | 35.89 [8.61–94.025] | 0.025 [0.0025–0.10] | 0.05 [0.00–0.137] |
| Sinopharm | 530.70 [209.10–1153.00] | 0.06 [0.02–0.25] | 0.10 [0.03–0.47] | 5.10 [2.69–15.92] | 0.000 [0.0000–0.000] | 0.01 [0.000–0.01] |
| p value | <0.0001 | 0.001 | <0.0001 | <0.0001 | 0.003 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ahmed, M.; Bellali, H.; Gdoura, M.; Zamali, I.; Kallala, O.; Ben Hmid, A.; Hamdi, W.; Ayari, H.; Fares, H.; Mechri, K.; et al. Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa). Vaccines 2022, 10, 1189. https://doi.org/10.3390/vaccines10081189
Ben Ahmed M, Bellali H, Gdoura M, Zamali I, Kallala O, Ben Hmid A, Hamdi W, Ayari H, Fares H, Mechri K, et al. Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa). Vaccines. 2022; 10(8):1189. https://doi.org/10.3390/vaccines10081189
Chicago/Turabian StyleBen Ahmed, Melika, Hedia Bellali, Mariem Gdoura, Imen Zamali, Ouafa Kallala, Ahlem Ben Hmid, Walid Hamdi, Hela Ayari, Hajer Fares, Karim Mechri, and et al. 2022. "Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa)" Vaccines 10, no. 8: 1189. https://doi.org/10.3390/vaccines10081189
APA StyleBen Ahmed, M., Bellali, H., Gdoura, M., Zamali, I., Kallala, O., Ben Hmid, A., Hamdi, W., Ayari, H., Fares, H., Mechri, K., Marzouki, S., Triki, H., Ben Alaya, N., Chahed, M. K., Klouz, A., Sebai Ben Amor, S., Ben Rayana, C., Razgallah Khrouf, M., Hamouda, C., ... Trabelsi, A. (2022). Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa). Vaccines, 10(8), 1189. https://doi.org/10.3390/vaccines10081189







