An Optimal Control Model to Understand the Potential Impact of the New Vaccine and Transmission-Blocking Drugs for Malaria: A Case Study in Papua and West Papua, Indonesia
Abstract
:1. Introduction
2. The Model and Parameter Estimation Result
2.1. The Model
- 1.
- Susceptible without vaccine () consists of a group of individuals susceptible to malaria who have not received a pre-erythrocytic vaccine yet.
- 2.
- Susceptible with a vaccine () consists of a group of individuals who are also susceptible to malaria but have already received a pre-erythrocytic vaccine.
- 3.
- Exposed without vaccine consists of a group of newly infected individuals from who have not yet gotten the pre-erythrocytic vaccine. We assume that individuals in this compartment are in the leaver stage. Hence, although these individuals do not show any symptoms yet, we assume that they can transmit Plasmodium to susceptible mosquitoes.
- 4.
- Exposed with vaccine consists of a group of newly infected individuals from . Although these individuals have already gotten vaccinated, the description is still the same with .
- 5.
- Infected consists of a group of individuals who have already gotten infected by malaria and show their symptoms.
- 6.
- Infected individuals undergo transmission-blocking treatment , defined as a group of individuals who already get infected, show symptoms, but get a transmission-blocking treatment. We assume that this treatment can kill the sexual Plasmodium (gametocytes).
- 7.
- Recovered but carrier consists of individuals who recovered from malaria (do not show symptoms anymore) and have a temporal immunity but still have asexual Plasmodium inside their bodies. Hence, this group of individuals can still transmit Plasmodium to the mosquito.
- 8.
- Fully recovered consists of a group of individuals who recovered from malaria and succeeded in the transmission-blocking treatment process. Hence, unlike in , individuals in lack sexual and asexual Plasmodium in their blood. Therefore, compartment does not transmit malaria anymore.
- 1.
- The rate of new individuals only came from newborns with a constant rate of . We ignore migration from our model.
- 2.
- Vertical transmission is neglected [31].
- 3.
- The infected individuals in and are capable to transmit Plasmodium to mosquitoes with a constant rate .
- 4.
- The pre-erythrocytic vaccine is given to individuals with a constant rate to give temporal protection from mosquito bites that can lead to malaria infection. Hence, we assume that the transmission rate of is less than .
- 5.
- The pre-erythrocytic vaccine is not for a lifetime. Hence, after a period of , individuals in will return to .
- 6.
- The transmission-blocking treatment is given to individuals in I with a constant rate to cure malaria and wipe out sexual and asexual Plasmodium from their blood.
- 7.
- The transmission-blocking treatment is not always successful in curing infected individuals.
- 8.
- The description of all parameters is given in Table 1 and assumed to be nonnegative.
- 1.
- Cost to implement pre-erythtocytic vaccine. The total cost to implement the pre-erythrocytic vaccine is given bywhere is the weight parameter for pre-erythrocytic parameter and is the final time of simulation. The unit of is . In this article, we consider the non-linearity term on this cost term as authors in [18,26].
- 2.
- Cost to implement transmission-blocking treatment. Similar to , we also use a quadratic term for . Hence, the total cost of transmission blocking is given bywhere is the weight parameter for transmission blocking. The unit of is .
- 3.
- Cost related to the high number of infected individuals. Except the cost related to the implementation of pre-erythrocytic and transmission-blocking, the cost for malaria control strategy also comes from the cost related to the high number of infected individuals who were not treated (I and ). This cost is given bywhere and are the weight parameters for I and , respectively. and respectively denote the cost associated with the high number of infected individuals, such as for maintaining health campaigns or any other related cost which comes as a consequence of the high number of infected individuals. The unit of and is .
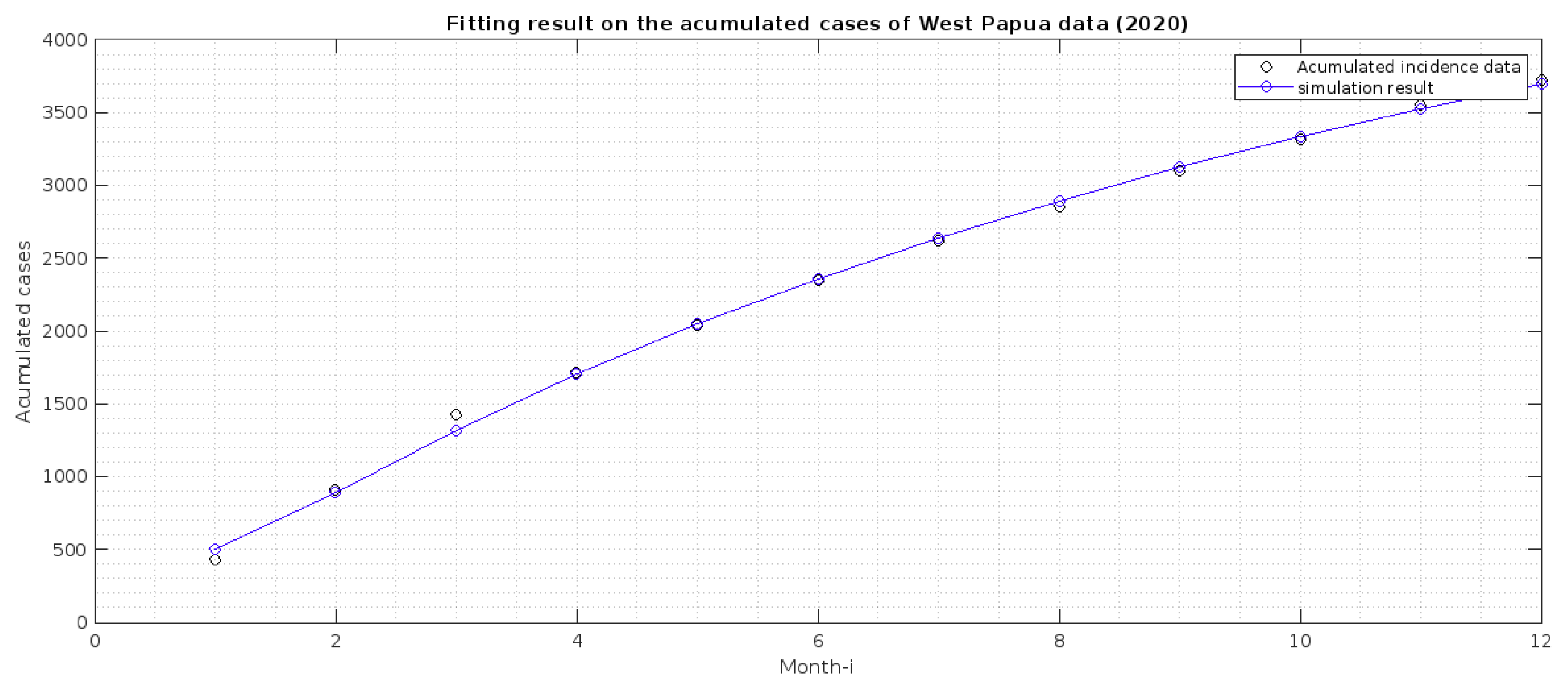
2.2. Parameter Estimation
- 1.
- Papua is more malaria-endemic than West Papua given the non-controlled basic reproduction number ( and ). See the formula of non-controlled basic reproduction number () in (12).
- 2.
- Based on the value of p and q, we conclude that individuals in Papua have a slightly bigger chance of reaching the malaria elimination target if there is a continuous implementation of treatment efforts compared to West Papua.
- 3.
- The infection rates from mosquitoes to humans and in West Papua is higher than in Papua. Hence, it is important to develop a media campaign that targets reducing the contact between humans and mosquitoes in West Papua than in Papua. Such campaigns may include the use of bed nets or mosquito repellent.
3. Dynamical Analysis of the Model
3.1. Preliminary Results on the Positiveness and Boundedness of the Solution
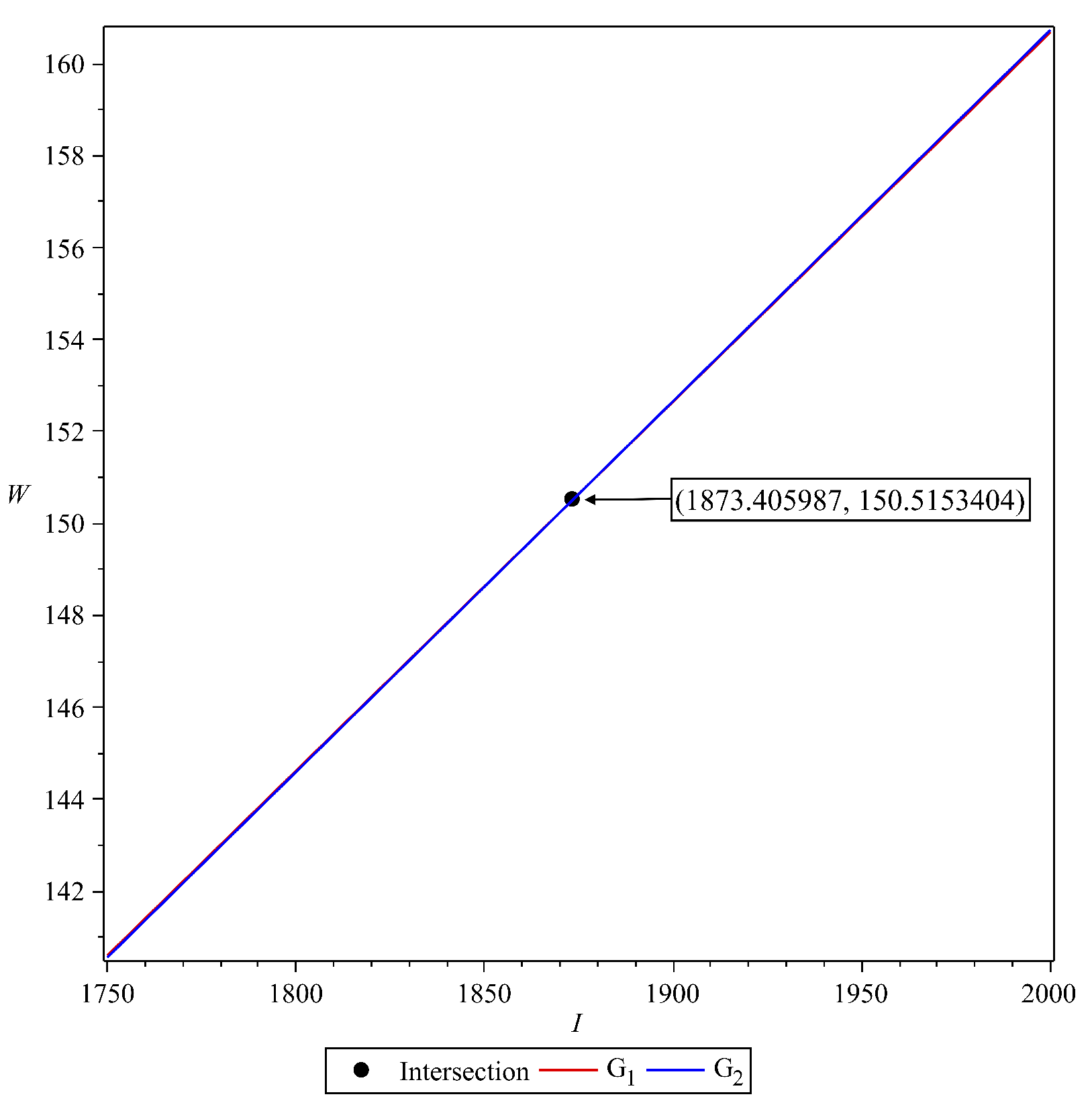
3.2. The Malaria-Free Equilibrium and the Controlled Reproduction Number
- 1.
- shows the path of transmission for a new case in mosquito due to a bite from susceptible mosquitoes to an exposed human in compartment .
- 2.
- shows the transmission path which gives a new infection in humans without pre-erythrocytic vaccine due to a bite from infected mosquitoes.
- 3.
- presents the transmission path for a new infection in the mosquito population after they bite exposed humans in .
- 4.
- presents the transmission path for a new infection in vaccinated human due to a bite from infected mosquitoes.
- 5.
- shows a transmission for a new infection in the mosquitoes population after biting an infected individual in I.
- 6.
- presents a transmission path for a new cases in I due to progression of and .
- 7.
- presents a transmission path for a new case in the mosquitoes population after biting an infected individual who is undergoing treatment .
- 8.
- presents a transmission path for a new case in treated human population due to the treatment rate from I.
- 9.
- presents a transmission path for a new case in the mosquito population after biting individuals in .
- 10.
- presents a transmission path for new cases in the compartment of humans who partially succeed in conducting transmission-blocking treatment.
3.3. The Malaria-Endemic Equilibrium
4. Sensitivity Analysis
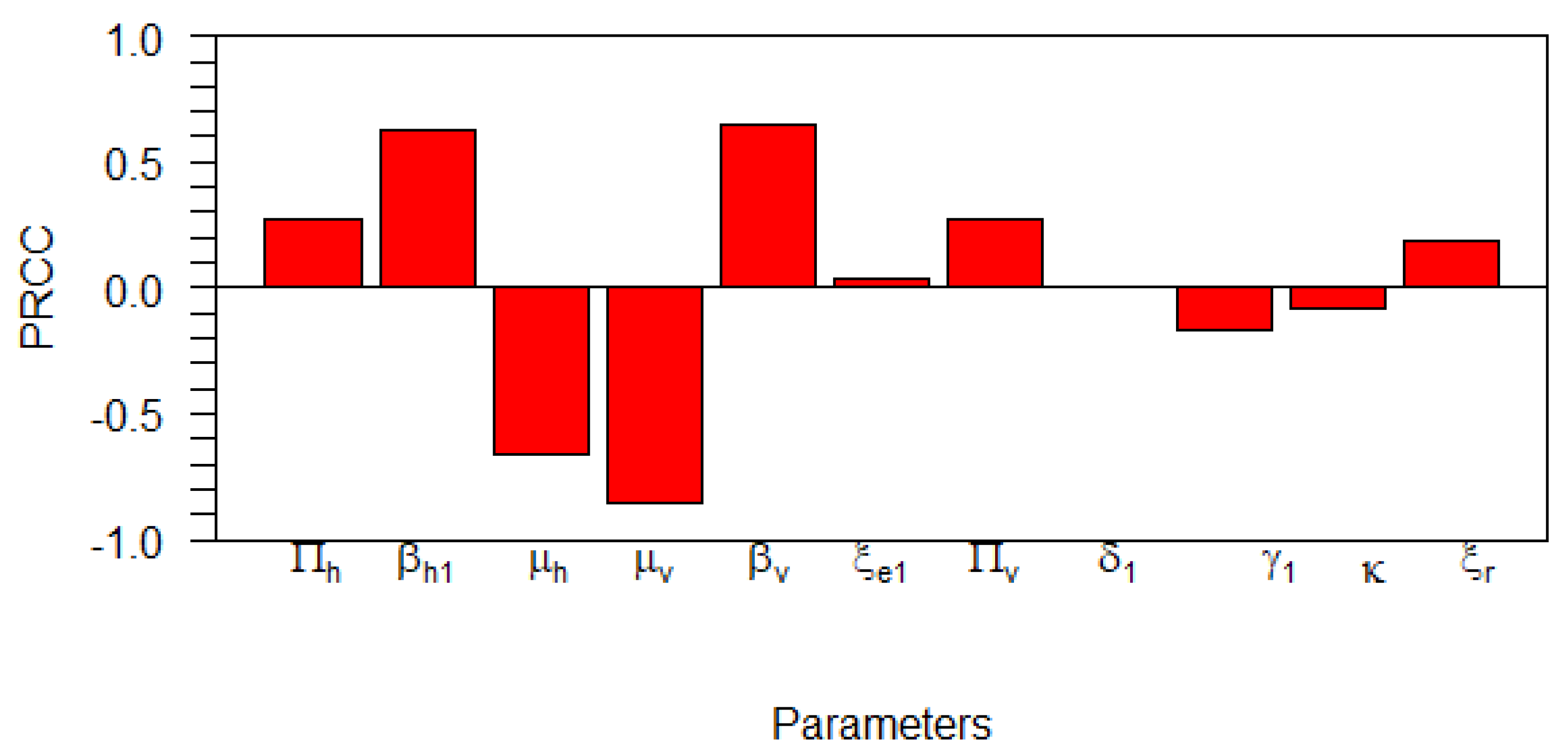
4.1. Global Sensitivity Analysis on the Basic Reproduction Number
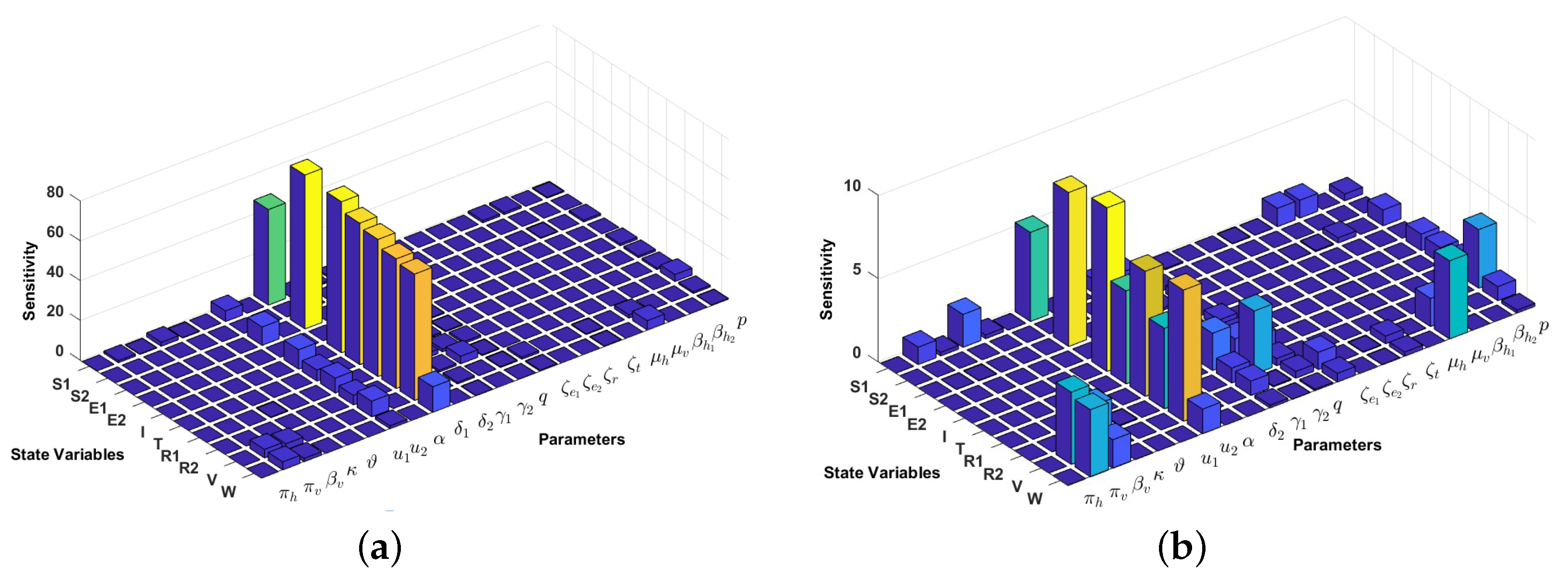
4.2. Local Sensitivity Analysis on the Model Variables
5. Existence of Solution and Characterization of the Optimal Control Problem
5.1. Existence of the Optimal Solution
- 1.
- The admissible control parameters and each model state variable are non-empty.
- 2.
- Control is convex and bounded.
- 3.
- Right hand side of our system is continuous and bounded above by a linear function in the state variables and the control parameters.
- 4.
- The integrand of is concave on
- 5.
- There exists positive constants and such that satisfies
- 1.
- Applying the methodology of Theorem 9.2.1 on page 182 of [57], the first criteria is fulfilled as the solutions of our model system exist and are bounded in as also shown by the existence of the super-solutions.
- 2.
- Using the definition of we have that set as bounded and closed.
- 3.
- 4.
- Suppose the objective functionand we set Then Additionally, let for then we have that and Thus,From (2), we observe thatFollowing this observation, we can then generalize that given any for we obtain Hence, the integrand of is concave.
- 5.
- Following the fact that the model state variable in the objective function, that is, I and are bounded as shown in 3. Above, there exits positive constants such that the sum of If we set we have thatin which This implies that there is a positive constant and a such that the integrand component of satisfies
5.2. Characterization of the Optimal Control Problem
6. Numerical Simulation of the Optimal Control Problem
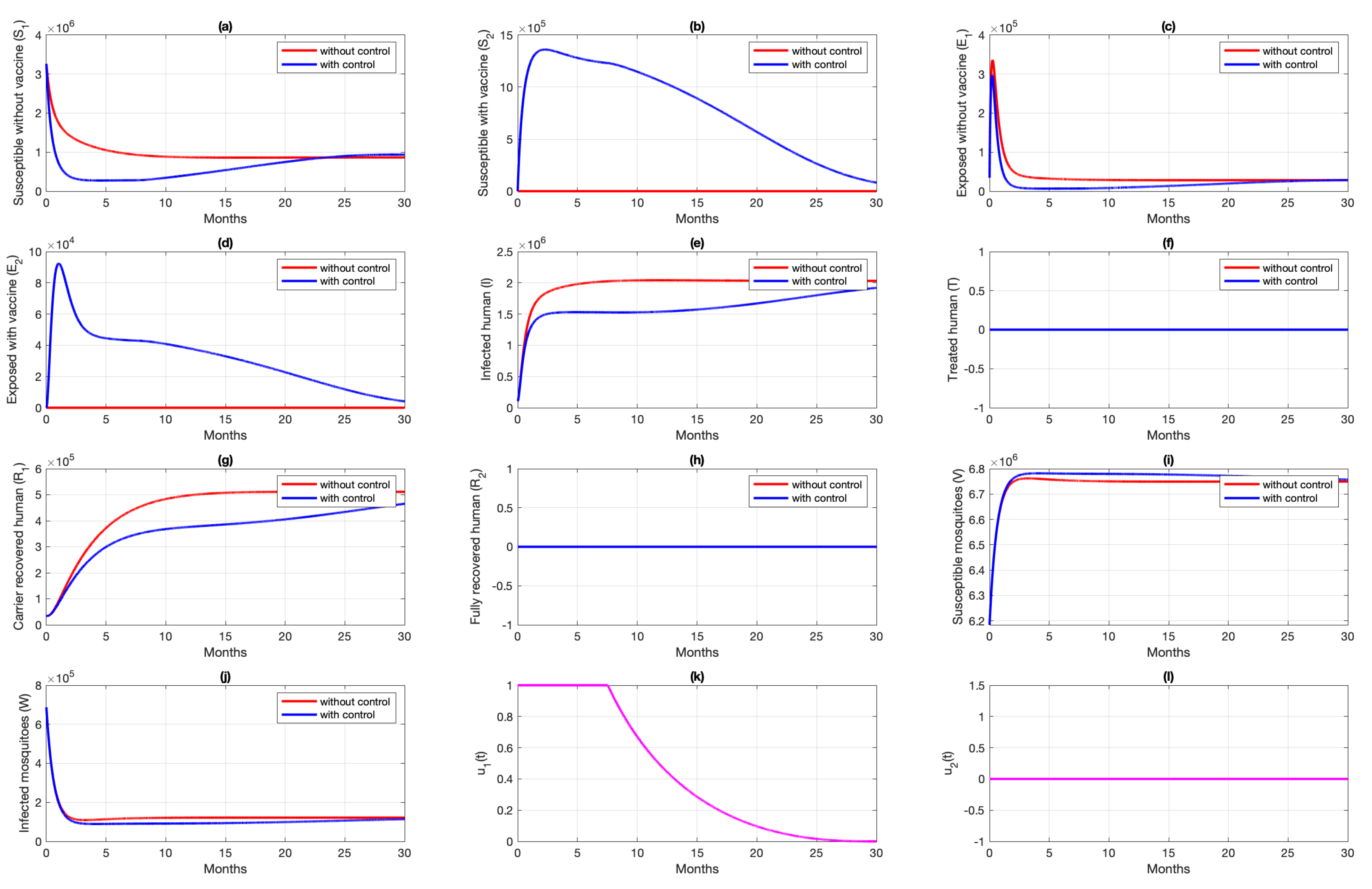
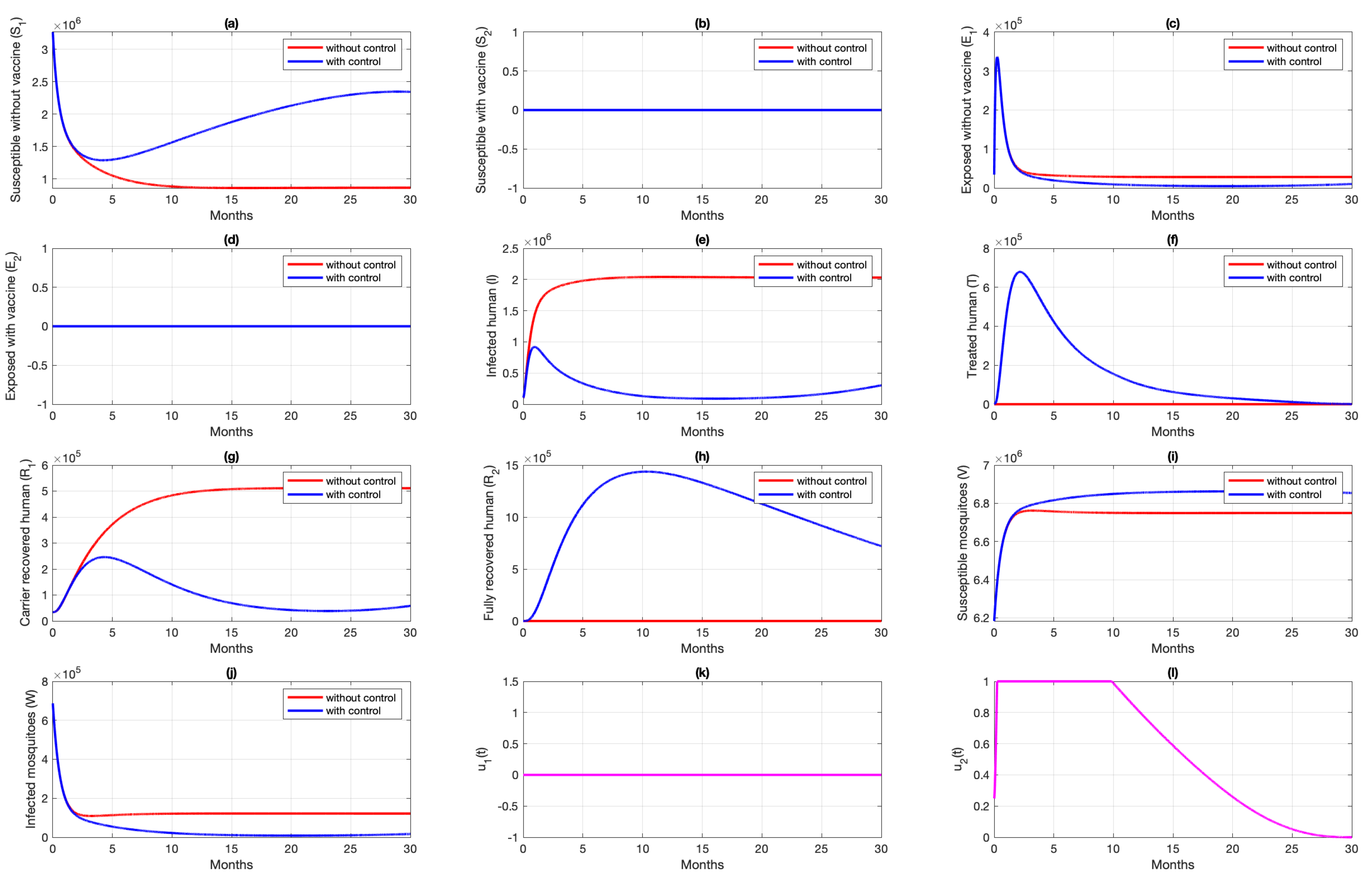
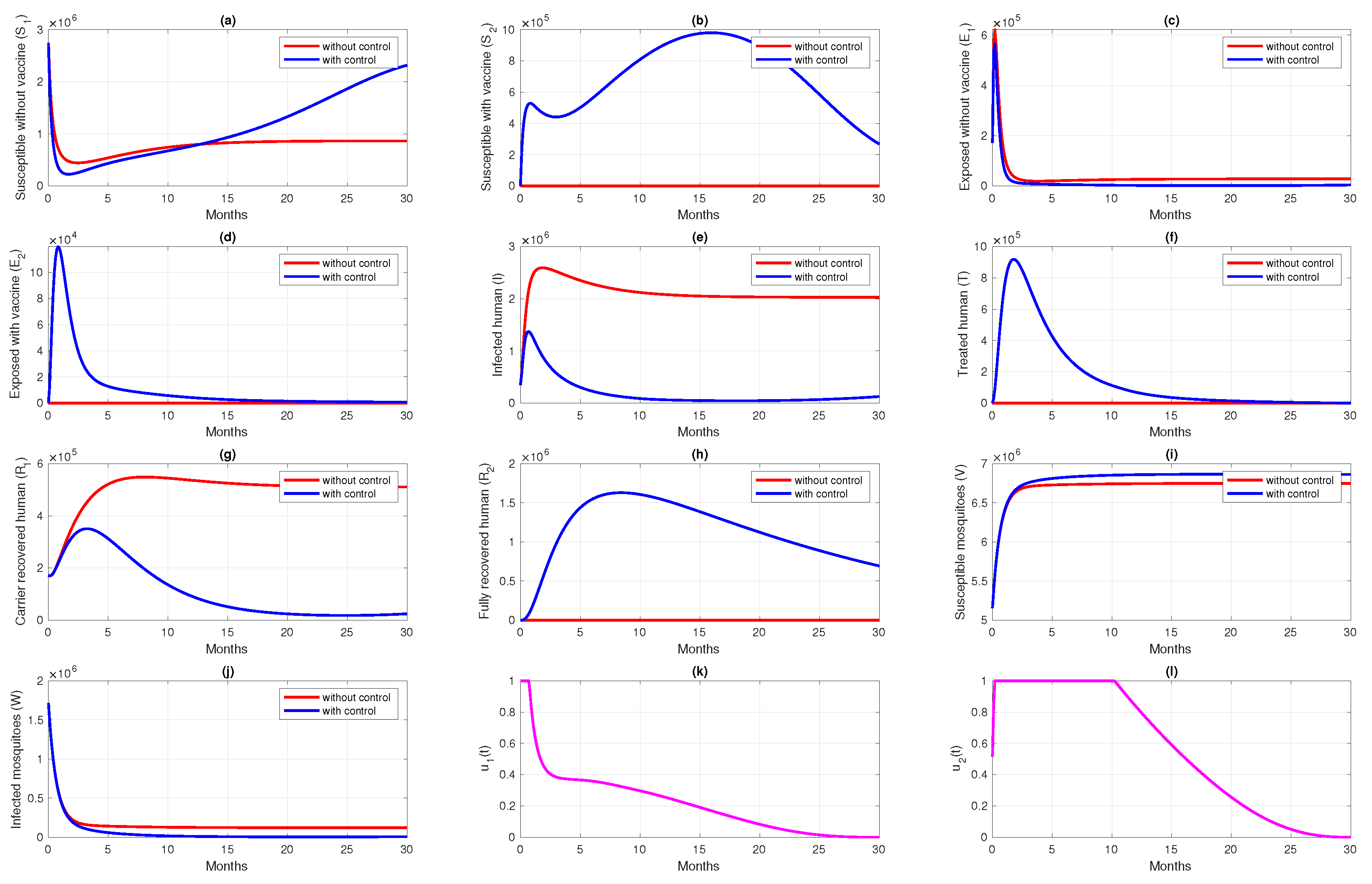
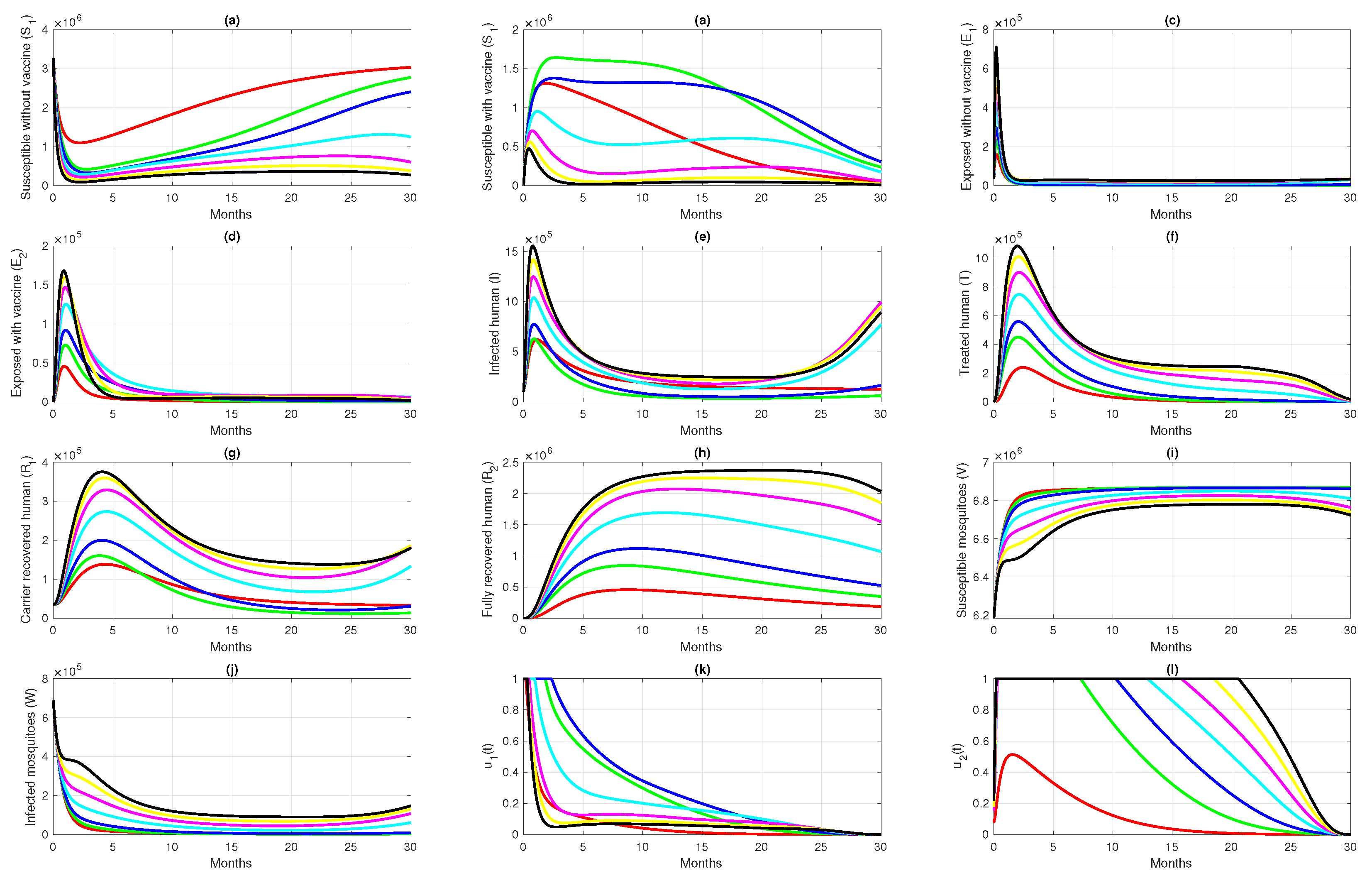
6.1. Different Combination of Interventions
6.2. Different Initial Condition of Population
6.3. Different Initial Basic Reproduction Number
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 20 January 2022).
- Malaria. Available online: https://www.who.int/health-topics/malaria#tab=tab_1 (accessed on 20 January 2022).
- CDC in Indonesia. Available online: https://www.cdc.gov/globalhealth/countries/indonesia/default.htm#malaria (accessed on 20 January 2022).
- About Malaria, Frequently Asked Questions. Available online: https://www.cdc.gov/malaria/about/faqs.html (accessed on 20 January 2022).
- Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 20 January 2022).
- Larval Control and Other Vector Control Interventions. Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/vector_control.html (accessed on 20 February 2022).
- Zheng, J.; Pan, H.; Gu, Y.; Zuo, X.; Ran, N.; Yuan, Y.; Zhang, C.; Wang, F. Prospects for Malaria Vaccines: Pre-erythrocytic Stages, Blood Stages, and Transmission-Blocking Stages. BioMed Res. Int. 2019, 2019, 9751471. [Google Scholar] [CrossRef]
- Duffy, P.E.; Gorres, J.P. Malaria vaccines since 2000: Progress, priorities, products. npj Vaccines 2020, 5, 48. [Google Scholar] [CrossRef]
- Ross, R. The Prevention of Malaria; John Murray: London, UK, 1911. [Google Scholar]
- Macdonald, G. The Epidemiology and Control of Malaria; Oxford University Press: Oxford, UK, 1957. [Google Scholar]
- Aron, J.L.; May, R.M. The population dynamics of malaria. In The Population Dynamics of Infectious Diseases: The Theory and Applications; Anderson, R.M., Ed.; Chapman and Hall: London, UK, 1982; pp. 139–179. [Google Scholar]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Okosun, K.O.; Rachid, O.; Marcus, N. Optimal control strategies and cost-effectiveness analysis of a malaria model. Biosystems 2013, 111, 83–101. [Google Scholar] [CrossRef]
- Prosper, O.; Ruktanonchai, N.; Martcheva, M. Optimal vaccination and bed net maintenance for the control of malaria in a region with naturally acquired immunity. J. Theor. Biol. 2014, 352, 142–156. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Churcher, T.S.; Gnai, A.C. Vaccine approaches to malaria control and elimination: Insights from mathematical models. Vaccine 2015, 33, 7544–7550. [Google Scholar] [CrossRef] [Green Version]
- Woldegerima, W.A.; Ouifki, R.; Banasiak, J. Mathematical analysis of the impact of transmission-blocking drugs on the population dynamics of malaria. Appl. Math. Comput. 2021, 400, 126005. [Google Scholar] [CrossRef]
- Kuddus, M.A.; Rahman, A. Modelling and analysis of human–mosquito malaria transmission dynamics in Bangladesh. Math. Comput. Simul. 2022, 193, 123–138. [Google Scholar] [CrossRef]
- Aldila, D.; Angelina, M. Optimal control problem and backward bifurcation on malaria transmission with vector bias. Heliyon 2021, 7, e06824. [Google Scholar] [CrossRef]
- Beretta, E.; Capasso, V.; Garao, D.G. A mathematical model for malaria transmission with asymptomatic carriers and two age groups in the human population. Math. Biosci. 2018, 300, 87–101. [Google Scholar] [CrossRef]
- Forouzannia, F.; Gumel, A.B. Mathematical analysis of an age-structured model for malaria transmission dynamics. Math. Biosci. 2014, 247, 80–94. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Fu, R.; Luo, J. Global analysis of a mathematical model on malaria with competitive strains and immune responses. Appl. Math. Comput. 2015, 259, 132–152. [Google Scholar] [CrossRef]
- Fatmawati; Tasman, H.; Purwati, U.D.; Herdicho, F.F.; Chukwu, C.W. An optimal control problem of malaria model with seasonality effect using real data. Commun. Math. Biol. Neurosci. 2021, 2021, 66. [Google Scholar]
- Fatmawati; Herdicho, F.F.; Windarto; Chukwu, C.W.; Tasman, H. An optimal control of malaria transmission model with mosquito seasonal factor. Results Phys. 2021, 25, 104238. [Google Scholar] [CrossRef]
- Tchoumi, S.Y.; Diagne, M.L.; Rwezaura, H.; Tchuenche, J.M. Malaria and COVID-19 co-dynamics: A mathematical model and optimal control. Appl. Math. Model. 2021, 99, 294–327. [Google Scholar] [CrossRef]
- Ndii, M.Z.; Adi, Y.A. Understanding the effects of individual awareness and vector controls on malaria transmission dynamics using multiple optimal control. Chaos Solitons Fractals 2021, 153, 111476. [Google Scholar] [CrossRef]
- Handari, B.D.; Vitra, F.; Ahya, R.; Nadya S., T.; Aldila, D. Optimal control in a malaria model: Intervention of fumigation and bed nets. Adv. Differ. Equ. 2019, 2019, 497. [Google Scholar] [CrossRef]
- Okosun, K.O.; Ouifki, R.; Marcus, N. Optimal control analysis of a malaria disease transmission model that includes treatment and vaccination with waning immunity. Bio Syst. 2011, 106, 136–145. [Google Scholar] [CrossRef]
- Zhao, R.; Mohammed-Awel, J. A mathematical model studying mosquito-stage transmission-blocking vaccines. Math. Biosci. Eng. 2014, 11, 1229–1245. [Google Scholar] [CrossRef]
- Marques-da-Silva, C.; Peissig, K.; Kurup, S.P. Pre-Erythrocytic Vaccines against Malaria. Vaccine 2020, 8, 400. [Google Scholar] [CrossRef]
- First Malaria Vaccine Receives Positive Scientific Opinion from EMA. Available online: https://www.ema.europa.eu/en/news/first-malaria-vaccine-receives-positive-scientific-opinion-ema (accessed on 14 October 2020).
- Fischer, P.R. Malaria and Newborns. J. Trop. Pediatr. 2003, 49, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Jumlah Penduduk Proyeksi (Jiwa) 2018–2020. Available online: https://papua.bps.go.id/indicator/12/277/1/jumlah-penduduk-proyeksi.html (accessed on 16 October 2021).
- Proyeksi Penduduk 2010–2020 (Jiwa) 2018–2020. Available online: https://papuabarat.bps.go.id/indicator/12/146/1/proyeksi-penduduk-2010-2020.html (accessed on 16 October 2021).
- Indikator Strategis Nasional. Available online: https://www.bps.go.id/QuickMap?id=0000000000 (accessed on 13 October 2021).
- About Malaria, Biology. Available online: https://www.cdc.gov/malaria/about/biology/index.html (accessed on 13 October 2021).
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Four-Year Efficacy of RTS,S/AS01E and Its Interaction with Malaria Exposure. N. Engl. J. Med. 2013, 368, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef] [Green Version]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar] [CrossRef]
- Lindblade, K.A.; Steinhardt, L.; Samuels, A.; Kachur, P.S.; Slutsker, L. The silent threat: Asymptomatic parasitemia and malaria transmission. Expert Rev. Anti-Infect. Ther. 2013, 11, 623–639. [Google Scholar] [CrossRef] [Green Version]
- Drakou, K.; Nikolaou, T.; Vasquez, M.; Petric, D.; Michaelakis, A.; Kapranas, A.; Papathedoulou, A.; Koliou, M. The effect of weather variables on mosquito activity: A snapshot of the main point of entry of Cyprus. Int. J. Environ. Res. Public Health 2020, 14, 1403. [Google Scholar] [CrossRef] [Green Version]
- Aldila, D. Optimal control for dengue eradication program under the media awareness effect. Int. J. Nonlinear Sci. Numer. Simul. 2021. [Google Scholar] [CrossRef]
- Aldila, D. Optimal control problem on COVID-19 disease transmission model considering medical mask, disinfectants and media campaign. E3S Web Conf. 2020, 202, 12009. [Google Scholar] [CrossRef]
- Hafidh, E.P.; Aulida, N.; Handari, B.D.; Aldila, D. Optimal control problem from tuberculosis and multidrug resistant tuberculosis transmission model. AIP Conf. Proc. 2018, 2023, 020223. [Google Scholar]
- Malaria: The Highest Cause of Death in the World. Available online: https://www.malaria.id/en/profile (accessed on 7 February 2022).
- St. Laurent, B.; Supratman, S.; Asih, P.B.S.; Bretz, D.; Mueller, J.; Miller, H.C.; Baharuddin, A.; Surya, A.; Ngai, M.; Laihad, F.; et al. Behaviour and molecular identification of Anopheles malaria vectors in Jayapura district, Papua province, Indonesia. Malar. J. 2016, 15, 192. [Google Scholar] [CrossRef] [Green Version]
- Mukandavire, Z.; Gumel, A.B.; Garira, W.; Tchuenche, J.M. Mathematical analysis of a model for HIV-malaria co-infection. Math. Biosci. Eng. 2009, 6, 333–362. [Google Scholar]
- Wadi, I.; Nath, M.; Anvikar, A.R.; Singh, P.; Sinha, A. Recent advances in transmission-blocking drugs for malaria elimination. Future Med. Chem. 2019, 11, 3047–3088. [Google Scholar] [CrossRef]
- Martcheva, M. An Introduction to Mathematical Epidemiology; Springer: New York, NY, USA, 2015. [Google Scholar]
- Roop-O, P.; Chinviriyasit, W.; Chinviriyasit, S. The effect of incidence function in backward bifurcation for malaria model with temporary immunity. Math. Biosci. 2015, 265, 47–64. [Google Scholar] [CrossRef]
- Chukwu, C.W.; Fatmawati. Modelling fractional-order dynamics of COVID-19 with environmental transmission and vaccination: A case study of Indonesia. AIMS Math. 2022, 7, 4416–4438. [Google Scholar] [CrossRef]
- Tsanou, B.; Kamgang, J.C.; Lubuma, J.M.-S.; Danga, D.E.H. Modeling pyrethroids repellency and its role on the bifurcation analysis for a bed net malaria model. Chaos Solitons Fractals 2020, 136, 109809. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.; Roberts, M.G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 2010, 7, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Marino, S.; Hogue, I.B.; Tay, C.J.; Kirschner, D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Chukwu, C.W.; Nyabadza, F. Mathematical Modeling of Listeriosis incorporating effects of awareness programs. Math. Mod. Comput. Simul. 2021, 13, 723–741. [Google Scholar] [CrossRef]
- Cesari, L. Optimization–Theory and Applications, Problems with Ordinary Differential Equations, Applications of Mathematics; Springer: New York, NY, USA, 1983. [Google Scholar]
- Fleming, W.H.; Rishel, R.W. Deterministic and Stochastic Optimal Control; Springer: New York, NY, USA, 1975. [Google Scholar]
- Lukes, D.L. Differential Equations: Classical to Controlled; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Pontryagin, L.S.; Boltyanskii, V.G.; Gamkrelize, R.V.; Mishchenko, E.F. The Mathematical Theory of Optimal Processes; Interscience Publishers (Division of John Wiley and Sons, Inc.): New York, NY, USA, 1962. [Google Scholar]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2007. [Google Scholar]





| Symbols | Biological Definitions | Papua | West Papua | Sources |
|---|---|---|---|---|
| Natural birth rate of humans | [32,33,34] | |||
| Natural birth rate of mosquitos | [32,33,35] | |||
| b | The average number of mosquitoes bite per unit time | estimated | ||
| The successful transmission rate of susceptible humans per bite | estimated | |||
| The successful transmission rate of susceptible humans who receive pre-erythrocytic vaccine per bite | estimated | |||
| Average infection rate to humans per unit time per mosquito | estimated | |||
| Average infection rate to humans who receive pre-erythrocytic vaccine per unit time per mosquito | estimated | |||
| Average of successful transmission rate of susceptible mosquitos per bite | estimated | |||
| Average infection rate to mosquitos per unit time per human | estimated | |||
| Waning rate of temporal immunity from recovered carriers | estimated | |||
| Waning rate of temporal immunity from fully recovered class | estimated | |||
| Vaccination rate with pre-erythrocytic vaccine | assumption | |||
| Rate of treatment with transmission-blocking drugs | estimated | |||
| pre-erythrocytic vaccine efficacy level | [36] | |||
| Waning rate of vaccine efficacy | [37] | |||
| Progression rate from exposed without vaccine class | estimated | |||
| Progression rate from exposed with vaccine class | 2 | 2 | [36,38] | |
| Natural recovery rate of infected humans by immune response | estimated | |||
| Duration of treatment with transmission-blocking drugs | estimated | |||
| p | Proportion of people in treatment who managed to get protection | estimated | ||
| q | Proportion of people in treatment who fail to receive protection and then recover naturally | estimated | ||
| Proportion of people in treatment who fail to receive protection and then return to the infected class | estimated | |||
| Correction factor for infection rate in mosquitoes by exposed humans | estimated | |||
| Correction factor for infection rate in mosquitoes by exposed humans with vaccine | assumption | |||
| Correction factor for infection rate in mosquitoes by recovered humans but carrier | estimated | |||
| Correction factor for infection rate in mosquitoes by humans in treatment | estimated | |||
| Natural death rate of humans | [34] | |||
| Natural death rate of mosquitos | [35] |
| Parameters | Interval Values | Sources |
|---|---|---|
| or | [32,33,34] | |
| or | [32,33,35] | |
| b | [27] | |
| [16,27] | ||
| [16,27,36] | ||
| or | [27,32,33] | |
| or | [27,32,33,36] | |
| [27] | ||
| or | [27,32,33] | |
| [38] | ||
| [38] | ||
| varied | ||
| varied | ||
| [36] | ||
| [37] | ||
| [38] | ||
| [36,38] | ||
| [46] | ||
| [47] | ||
| p | varied | |
| q | varied | |
| varied | ||
| [16] | ||
| varied | ||
| [16] | ||
| [16] | ||
| [34] | ||
| [35] |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| 2 | |||
| p | |||
| q | |||
| Parameter | PRCC Values | p-Values | Significant? |
|---|---|---|---|
| 0 | TRUE | ||
| 0 | TRUE | ||
| 0 | TRUE | ||
| 0 | TRUE | ||
| 0 | TRUE | ||
| FALSE | |||
| 0 | TRUE | ||
| FALSE | |||
| TRUE | |||
| FALSE | |||
| TRUE |
| Case | Scenario for , and | Inverted Case | Cost | Colour | |
|---|---|---|---|---|---|
| 1 | Reduced 50% from case 3 | 1.005 | 52,787 | Red | |
| 2 | Reduced 25% from case 3 | 1.508 | 129,702 | Green | |
| 3 | As in Table 1 for Papua data | 2.011 | 170,680 | Blue | |
| 4 | Increased 50% from case 3 | 3.016 | 185,168 | Cyan | |
| 5 | Increased 100% from case 3 | 4.022 | 183,584 | Magenta | |
| 6 | Increased 150% from case 3 | 5.027 | 184,294 | Yellow | |
| 7 | Increased 200% from case 3 | 6.033 | 185,505 | Black |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handari, B.D.; Ramadhani, R.A.; Chukwu, C.W.; Khoshnaw, S.H.A.; Aldila, D. An Optimal Control Model to Understand the Potential Impact of the New Vaccine and Transmission-Blocking Drugs for Malaria: A Case Study in Papua and West Papua, Indonesia. Vaccines 2022, 10, 1174. https://doi.org/10.3390/vaccines10081174
Handari BD, Ramadhani RA, Chukwu CW, Khoshnaw SHA, Aldila D. An Optimal Control Model to Understand the Potential Impact of the New Vaccine and Transmission-Blocking Drugs for Malaria: A Case Study in Papua and West Papua, Indonesia. Vaccines. 2022; 10(8):1174. https://doi.org/10.3390/vaccines10081174
Chicago/Turabian StyleHandari, Bevina D., Rossi A. Ramadhani, Chidozie W. Chukwu, Sarbaz H. A. Khoshnaw, and Dipo Aldila. 2022. "An Optimal Control Model to Understand the Potential Impact of the New Vaccine and Transmission-Blocking Drugs for Malaria: A Case Study in Papua and West Papua, Indonesia" Vaccines 10, no. 8: 1174. https://doi.org/10.3390/vaccines10081174
APA StyleHandari, B. D., Ramadhani, R. A., Chukwu, C. W., Khoshnaw, S. H. A., & Aldila, D. (2022). An Optimal Control Model to Understand the Potential Impact of the New Vaccine and Transmission-Blocking Drugs for Malaria: A Case Study in Papua and West Papua, Indonesia. Vaccines, 10(8), 1174. https://doi.org/10.3390/vaccines10081174








