High SARS-CoV-2 Seroprevalence and Rapid Neutralizing Antibody Decline among Agricultural Workers in Rural Guatemala, June 2020–March 2021
Abstract
:1. Introduction
2. Methods
3. SARS-CoV-2 Antibody Analysis
4. Statistical Analyses
5. Ethical Oversight
6. Results
7. Risk Factors for Enrollment SARS-CoV-2 Seropositivity
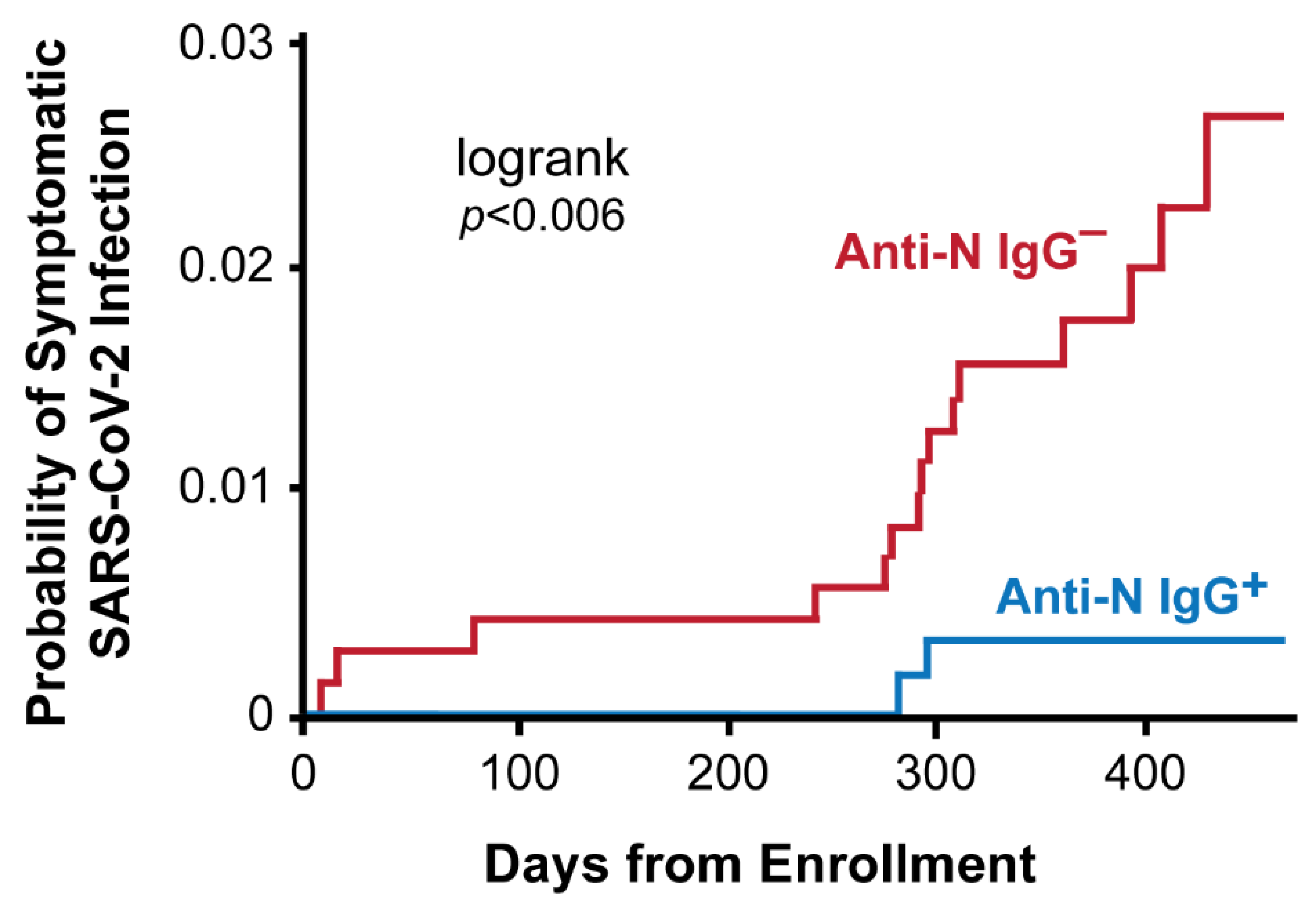
8. Risk of COVID-19 by SARS-CoV-2 Nucleocapsid IgG Serostatus
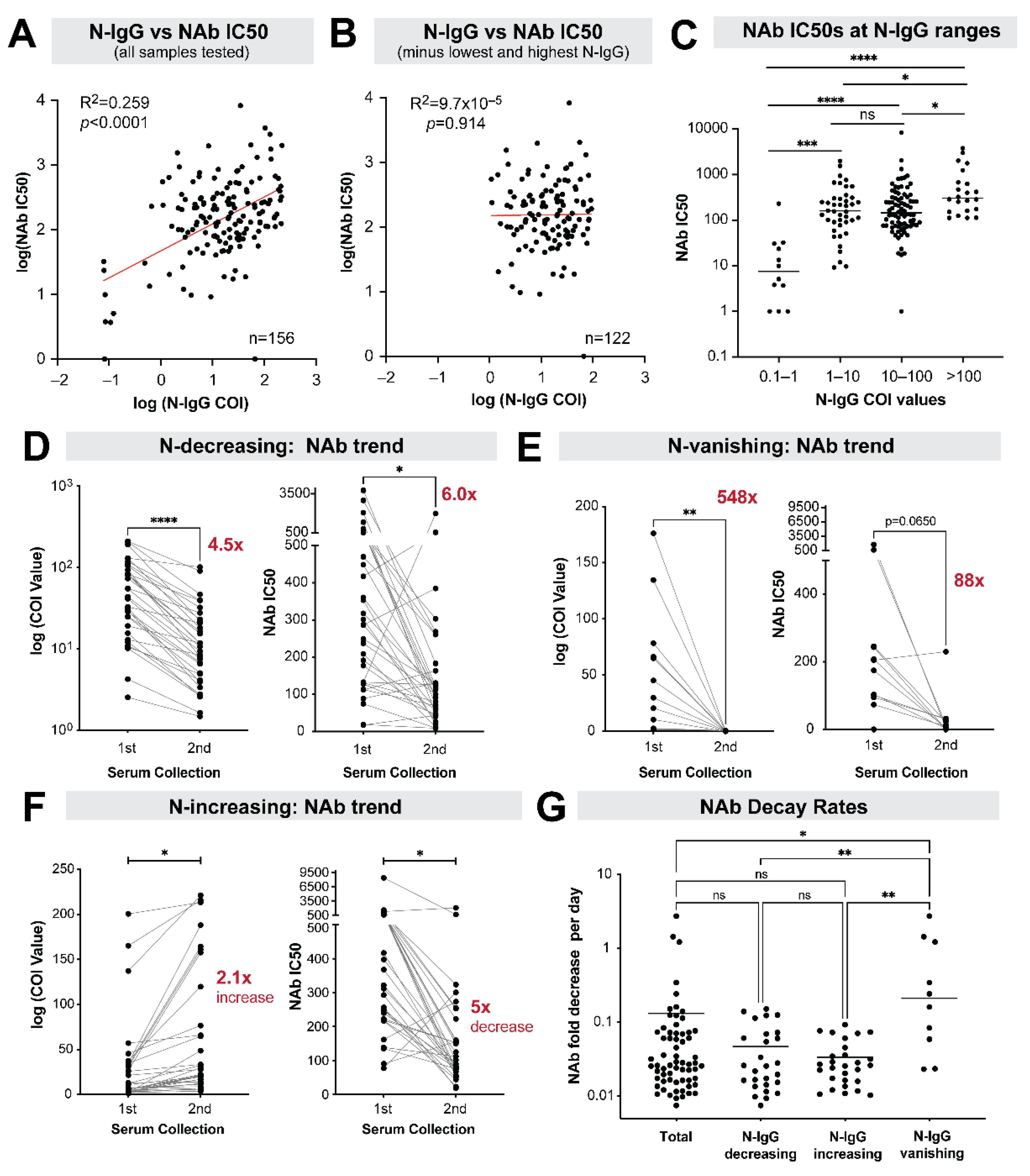
9. Stability of Neutralizing Antibody Responses among Nucleocapsid IgG Positive Individuals
10. Subgroup Analysis of Antibody Neutralization Decay
11. Discussion
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chicas, R.; Xiuhtecutli, N.; Houser, M.; Glastra, S.; Elon, L.; Sands, J.M.; McCauley, L.; Hertzberg, V. COVID-19 and Agricultural Workers: A Descriptive Study. J. Immigr. Minor. Health 2022, 24, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Holshue, M.; Dostal, T.K.H.; Newman, L.P.; Lindquist, S. COVID-19 Outbreak Among Farmworkers—Okanogan County, Washington, May–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Brackbill, R.M.; Cameron, L.L.; Behrens, V. Prevalence of chronic diseases and impairments among US farmers, 1986–1990. Am. J. Epidemiol. 1994, 139, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Dally, M.; Butler-Dawson, J.; Cruz, A.; Krisher, L.; Johnson, R.J.; Asensio, C.; Pilloni, W.D.; Asturias, E.J.; Newman, L.S. Longitudinal trends in renal function among first time sugarcane harvesters in Guatemala. PLoS ONE 2020, 15, e0229413. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, F.; Hernandez, M.A.; Paz, C. Short-term impacts of COVID-19 on food security and nutrition in rural Guatemala: Phone-based farm household survey evidence. Agric. Econ. 2021, 52, 477–494. [Google Scholar] [CrossRef]
- Chen, Y.H.; Glymour, M.; Riley, A.; Balmes, J.; Duchowny, K.; Harrison, R.; Matthay, E.; Bibbins-Domingo, K. Excess mortality associated with the COVID-19 pandemic among Californians 18-65 years of age, by occupational sector and occupation: March through November 2020. PLoS ONE 2021, 16, e0252454. [Google Scholar] [CrossRef]
- Lusk, J.L.; Chandra, R. Farmer and farm worker illnesses and deaths from COVID-19 and impacts on agricultural output. PLoS ONE 2021, 16, e0250621. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Mora, A.M.; Nkwocha, O.; Kogut, K.; Rauch, S.A.; Morga, N.; Hernandez, S.; Wong, M.P.; Huen, K.; Andrejko, K.; et al. Prevalence and Clinical Profile of Severe Acute Respiratory Syndrome Coronavirus 2 Infection among Farmworkers, California, USA, June–November 2020. Emerg. Infect. Dis. 2021, 27, 1330–1342. [Google Scholar] [CrossRef]
- Mora, A.M.; Lewnard, J.A.; Kogut, K.; Rauch, S.A.; Hernandez, S.; Wong, M.P.; Huen, K.; Chang, C.; Jewell, N.P.; Holland, N.; et al. Risk Factors Associated With SARS-CoV-2 Infection Among Farmworkers in Monterey County, California. JAMA Netw. Open 2021, 4, e2124116. [Google Scholar] [CrossRef]
- CDC. Interim Guidelines for COVID-19 Antibody Testing 24 January 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 23 March 2022).
- Petersen, L.R.; Sami, S.; Vuong, N.; Pathela, P.; Weiss, D.; Morgenthau, B.M.; Henseler, R.A.; Daskalakis, D.C.; Atas, J.; Patel, A.; et al. Lack of Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a Large Cohort of Previously Infected Persons. Clin. Infect. Dis. 2021, 73, e3066–e3073. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Reed, C.; Lim, T.; Montgomery, J.M.; Klena, J.D.; Hall, A.J.; Fry, A.M.; Cannon, D.L.; Chiang, C.F.; Gibbons, A.; et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, 23 March–12 May 2020. JAMA Intern. Med. 2020, 180, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, 6529. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Tea, F.; Ospina Stella, A.; Aggarwal, A.; Ross Darley, D.; Pilli, D.; Vitale, D.; Merheb, V.; Lee, F.X.; Cunningham, P.; Walker, G.J.; et al. SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants. PLoS Med. 2021, 18, e1003656. [Google Scholar] [CrossRef]
- Olson, D.; Calvimontes, D.M.; Lamb, M.M.; Guzman, G.; Barrios, E.; Chacon, A.; Rojop, N.; Arias, K.; Gomez, M.; Bolanos, G.A.; et al. Clinical and Economic Impact of COVID-19 on Plantation Workers: Preliminary Results from the Guatemala Agricultural Workers and Respiratory Illness Impact (AGRI) Study. Emerging Infect. Dis. 2022, 28. [Google Scholar] [CrossRef]
- Olbrich, L.; Castelletti, N.; Schälte, Y.; Garí, M.; Pütz, P.; Bakuli, A.; Pritsch, M.; Kroidl, I.; Saathoff, E.; Noller, J.M.; et al. Head-to-head evaluation of seven different seroassays including direct viral neutralisation in a representative cohort for SARS-CoV-2. J. Gen. Virol. 2021, 102, 001653. [Google Scholar] [CrossRef]
- Olbrich, L.; Castelletti, N.; Schälte, Y.; Garí, M.; Pütz, P.; Bakuli, A.; Pritsch, M.; Kroidl, I.; Saathoff, E.; Noller, J.M.; et al. Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e01694-20. [Google Scholar]
- Hasenkrug, K.J.; Feldmann, F.; Myers, L.; Santiago, M.L.; Guo, K.; Barrett, B.S.; Mickens, K.L.; Carmody, A.; Okumura, A.; Rao, D.; et al. Recovery from Acute SARS-CoV-2 Infection and Development of Anamnestic Immune Responses in T Cell-Depleted Rhesus Macaques. mBio 2021, 12, e0150321. [Google Scholar] [CrossRef]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.H.; Michailidis, E.; Lorenzi, J.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.; Barrett, B.S.; Mickens, K.L.; Vladar, E.K.; Morrison, J.H.; Hasenkrug, K.J.; Poeschla, E.M.; Santiago, M.L. Interferon Resistance of Emerging SARS-CoV-2 Variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- WHO (Ed.) WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. In Expert Committee on Biological Standardization Geneva, 9–10 December 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Zou, G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- Yelland, L.N.; Salter, A.B.; Ryan, P. Performance of the Modified Poisson Regression Approach for Estimating Relative Risks from Clustered Prospective Data. Am. J. Epidemiol. 2011, 174, 984–992. [Google Scholar] [CrossRef]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef]
- Helfand, M.; Fiordalisi, C.; Wiedrick, J.; Ramsey, K.L.; Armstrong, C.; Gean, E.; Winchell, K.; Arkhipova-Jenkins, I. Risk for Reinfection After SARS-CoV-2: A Living, Rapid Review for American College of Physicians Practice Points on the Role of the Antibody Response in Conferring Immunity Following SARS-CoV-2 Infection. Ann. Intern. Med. 2022, 175, 547–555. [Google Scholar] [CrossRef]
- Jehn, M.; Pandit, U.; Sabin, S.; Tompkins, C.; White, J.; Kaleta, E.; Dale, A.P.; Ross, H.M.; Mac McCullough, J.; Pepin, S.; et al. Accuracy of Case-Based Seroprevalence of SARS-CoV-2 Antibodies in Maricopa County, Arizona. Am. J. Public Health 2022, 112, 38–42. [Google Scholar] [CrossRef]
- Dyal, J.W.; Grant, M.P.; Broadwater, K.; Bjork, A.; Waltenburg, M.A.; Gibbins, J.D.; Hale, C.; Silver, M.; Fischer, M.; Steinberg, J.; et al. COVID-19 Among Workers in Meat and Poultry Processing Facilities—19 States, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 557–561. [Google Scholar] [CrossRef]
- Walshe, N.; Fennelly, M.; Hellebust, S.; Wenger, J.; Sodeau, J.; Prentice, M.; Grice, C.; Jordan, V.; Comerford, J.; Downey, V.; et al. Assessment of Environmental and Occupational Risk Factors for the Mitigation and Containment of a COVID-19 Outbreak in a Meat Processing Plant. Front. Public Health 2021, 9, 769238. [Google Scholar] [CrossRef]
- Günther, T.; Czech-Sioli, M.; Indenbirken, D.; Robitaille, A.; Tenhaken, P.; Exner, M.; Ottinger, M.; Fischer, N.; Grundhoff, A.; Brinkmann, M.M. SARS-CoV-2 outbreak investigation in a German meat processing plant. EMBO Mol. Med. 2020, 12, e13296. [Google Scholar] [CrossRef]
- Puchades, A.; Daniel, R.; Geen, J.; Peden, J.; Lewis, H.; Nnoaham, K. SARS-CoV-2 sero-prevalence in the workforces of three large workplaces in South Wales: A sero-epidemiological study. BMC Public Health 2022, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- MSPAS Acuerdo Ministerial Número 146-2020. 2020. Available online: https://dgps-sso.mintrabajo.gob.gt/files/ACUERDO-MINISTERIAL-146-2020.pdf (accessed on 21 March 2022).
- Abedin, M.; Islam, M.A.; Rahman, F.N.; Reza, H.M.; Hossain, M.Z.; Arefin, A.; Hossain, A. Willingness to vaccinate against COVID-19 among Bangladeshi adults: Understanding the strategies to optimize vaccination coverage. PLoS ONE 2021, 16, e0250495. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, A.M.; Spicer, K.B.; Thoroughman, D.; Glick, C.; Winter, K. Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination—Kentucky, May–June 2021. MMWR Morb. Mortal Wkly Rep. 2021, 70, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Tyner, H.L.; Thompson, M.G.; Burgess, J.L.; Grant, L.; Gaglani, M.; Kuntz, J.L.; Naleway, A.L.; Thornburg, N.J.; Caban-Martinez, A.J.; Yoon, S.K.; et al. Neutralizing Antibody Response to Pseudotype SARS-CoV-2 Differs between mRNA-1273 and BNT162b2 COVID-19 Vaccines and by History of SARS-CoV-2 Infection. Clin. Infect. Dis. 2021, ciab1038. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity and mRNA Vaccine Effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: A Prospective Observational Study. medRxiv 2022. [Google Scholar] [CrossRef]
- Harrington, W.E.; Trakhimets, O.; Andrade, D.V.; Dambrauskas, N.; Raappana, A.; Jiang, Y.; Houck, J.; Selman, W.; Yang, A.; Vigdorovich, V.; et al. Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19. Cell Rep. Med. 2021, 2, 100253. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Zhu, F.; Ong, S.W.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Chia, W.N.; Zhu, F.; Ong, S.W.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J. Clin. Investig. 2021, 131, 17. [Google Scholar]
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review. Ann. Intern. Med. 2021, 174, 655–662. [Google Scholar] [CrossRef]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission from People without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Takahashi, S.; Hakim, J.; Kelly, J.D.; Torres, L.; Iyer, N.S.; Turcios, K.; Janson, O.; Munter, S.E.; Thanh, C.; et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci. Adv. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
| Demographics | |
|---|---|
| Age in years (mean, SD) | 31.2 (8.5) |
| Male sex, n (%) | 1125.0 (84.3) |
| Ethnicity, n (%): Ladino | 581.0 (43.6) |
| Indigenous | 92.0 (6.9) |
| Other * | 3.0 (0.2) |
| Don’t know | 658.0 (49.3) |
| Clinical Data | |
| Obesity (measured BMI> 30 kg/m2), n = 64 | |
| Class 1 (BMI 30–<35 kg/m2) | 54.0 (84.4) |
| Class 2 (BMI 35–<40 kg/m2) | 9.0 (14.1) |
| Class 3 (BMI ≥ 40 kg/m2) | 1.0 (1.5) |
| Kidney disease, n (%) | 46.0 (3.5) |
| Blood disorder (e.g., anemia), n (%) | 21.0 (1.6) |
| Cardiovascular disease (e.g., heart failure, CAD), n (%) | 19.0 (1.4) |
| Diabetes, n (%) | 13.0 (1.0) |
| Liver disease, n (%) | 15.0 (1.1) |
| Asthma, n (%) | 8.0 (0.6) |
| Pulmonary disease (e.g., COPD), n (%) | 6.0 (0.5) |
| Neurologic disease (e.g., stroke), n (%) | 8.0 (0.6) |
| Taking medications (Rx or OTC), n (%) | 160.0 (12.0) |
| Ever received influenza vaccine, n (%) | 75.0 (5.6) |
| ILI symptoms at enrollment, n (%) | 33.0 (2.5) |
| Cough | 23.0 (1.7) |
| Fever | 13.0 (1.0) |
| SARS-CoV-2 IgG positive at enrollment, n (%) | 616.0 (46.2) |
| Work Conditions | |
| Type of work, n (%) | |
| Administration | 9.0 (0.7) |
| Field worker | 947.0 (71.0) |
| Field manager | 48.0 (3.6) |
| Packer (plant worker) | 317.0 (23.8) |
| Plant manager | 13.0 (1.0) |
| Individual monthly income, $USD, median (IQR) | 337.7 (311.7, 376.6) |
| Household Conditions | |
| # of household members, median (IQR) | 5.0 (4, 7) |
| # adults in household, median (IQR) | 3 (2,4) |
| # children in household, median (IQR) | 2 (1,3) |
| Household monthly income, $USD, median (IQR) | 363.6 (311.7, 454.5) |
| Risk Factor | Unadjusted Relative Risk (95% CI) | p-Value | Adjusted Relative Risk (95% CI) * | p-Value |
|---|---|---|---|---|
| Age (years) | 1.00 (0.99–1.01) | 0.39 | 1.00 (0.99–1.01) | 0.69 |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 1.45 (1.26–1.66) | <0.0001 | 0.95 (0.87–1.04) | 0.27 |
| Ethnicity | ||||
| Ladino | Ref | Ref | ||
| Indigenous | 1.23 (1.02–1.47) | 0.03 | 1.12 (0.99–1.26) | 0.06 |
| Unknown | 1.08 (0.98–1.19) | 0.13 | 1.06 (0.98–1.15) | 0.17 |
| Number of household members | 1.02 (1.01–1.03) | <0.0001 | 1.01 (1.00–1.02) | 0.04 |
| Number of adults in household | 1.03 (1.00–1.06) | 0.06 | - | - |
| Number of children in household | 1.02 (1.00–1.05) | 0.08 | - | - |
| At least 1 comorbid health condition | 1.04 (0.90–1.20) | 0.60 | - | - |
| Kidney disease | 0.90 (0.71–1.12) | 0.34 | - | - |
| Blood disorder (e.g., anemia) | 0.99 (0.68–1.44) | 0.96 | - | - |
| Cardiovascular disease (e.g., heart failure, CAD) | 1.30 (0.97–1.75) | 0.08 | 1.13 (0.87–1.47) | 0.37 |
| Diabetes | 0.68 (0.38–1.23) | 0.21 | - | - |
| Liver disease | 1.57 (1.22–2.02) | <0.001 | 1.26 (0.91–1.75) | 0.17 |
| Asthma | 1.42 (1.02–1.96) | 0.04 | 1.22 (0.97–1.53) | 0.10 |
| Pulmonary disease (e.g., COPD) | 1.11 (0.62–1.99) | 0.73 | - | - |
| Neurologic disease (e.g., stroke) | 1.31 (0.81–2.11) | 0.27 | - | - |
| Obesity | 1.18 (0.86–1.64) | 0.31 | - | - |
| ILI symptoms within 10 days of enrollment | 1.11 (0.88–1.41) | 0.37 | - | - |
| Cough | 1.31 (1.08–1.59) | <0.01 | 1.28 (1.13–1.46) | <0.001 |
| Fever | 0.84 (0.53–1.32) | 0.44 | - | - |
| Type of work | ||||
| Field worker | Ref | - | ||
| Administration | 0.59 (0.22–1.57) | 0.29 | 0.61 (0.22–1.65) | 0.33 |
| Field manager | 0.88 (0.61–1.27) | 0.50 | 0.88 (0.61–1.28) | 0.51 |
| Packer (plant worker) | 1.98 (1.64–2.39) | <0.0001 | 2.00 (1.67–2.38) | <0.0001 |
| Plant manager | 1.86 (1.41–2.46) | <0.0001 | 1.82 (1.36–2.43) | <0.0001 |
| Individual monthly income, $USD (per $100 USD) | 1.02 (0.97–1.08) | 0.47 | - | - |
| Household monthly income, $USD (per $100 USD) | 1.02 (0.98–1.05) | <0.36 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, C.; Lesteberg, K.E.; Lamb, M.M.; Calvimontes, D.M.; Guo, K.; Barrett, B.S.; Mickens, K.L.; Duca, L.M.; Monzon, J.; Chard, A.N.; et al. High SARS-CoV-2 Seroprevalence and Rapid Neutralizing Antibody Decline among Agricultural Workers in Rural Guatemala, June 2020–March 2021. Vaccines 2022, 10, 1160. https://doi.org/10.3390/vaccines10071160
Iwamoto C, Lesteberg KE, Lamb MM, Calvimontes DM, Guo K, Barrett BS, Mickens KL, Duca LM, Monzon J, Chard AN, et al. High SARS-CoV-2 Seroprevalence and Rapid Neutralizing Antibody Decline among Agricultural Workers in Rural Guatemala, June 2020–March 2021. Vaccines. 2022; 10(7):1160. https://doi.org/10.3390/vaccines10071160
Chicago/Turabian StyleIwamoto, Chelsea, Kelsey E. Lesteberg, Molly M. Lamb, Diva M. Calvimontes, Kejun Guo, Bradley S. Barrett, Kaylee L. Mickens, Lindsey M. Duca, Jose Monzon, Anna N. Chard, and et al. 2022. "High SARS-CoV-2 Seroprevalence and Rapid Neutralizing Antibody Decline among Agricultural Workers in Rural Guatemala, June 2020–March 2021" Vaccines 10, no. 7: 1160. https://doi.org/10.3390/vaccines10071160
APA StyleIwamoto, C., Lesteberg, K. E., Lamb, M. M., Calvimontes, D. M., Guo, K., Barrett, B. S., Mickens, K. L., Duca, L. M., Monzon, J., Chard, A. N., Guzman, G., Barrios, E., Rojop, N., Arias, K., Gomez, M., Paiz, C., Bolanos, G. A., Edwards, K. M., Zielinski Gutierrez, E., ... Olson, D. (2022). High SARS-CoV-2 Seroprevalence and Rapid Neutralizing Antibody Decline among Agricultural Workers in Rural Guatemala, June 2020–March 2021. Vaccines, 10(7), 1160. https://doi.org/10.3390/vaccines10071160






