1. Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, international traveling was significantly limited. Although some countries closed borders, most other countries imposed a variety of entering requirements related to COVID-19, such as mandatory quarantine, additional insurances, proofs of vaccination and boosters, or negative polymerase chain reaction test (PCR)-tests for SARS-CoV-2 infection [
1,
2]. In addition to vaccination against SARS-CoV-2, the usual travel vaccinations continue to be required. Currently, international travel is increasing, including visits to tropical destinations [
3], back to pre-COVID-19 levels. International travel to destinations that require vaccination comprises considerable number of travelers. For example, in 2019, more than half a million travelers from the United States visited Africa [
4]. Travelers health, including acquiring the necessary vaccinations, is therefore an important issue.
Travel vaccines have been used for over 2 centuries [
5] and significantly reduce the risk of acquiring a variety of infection diseases [
6]. However, whether vaccinations are routine, required, and recommended depends on the travel destination, planned activities, and duration of the stay [
7].
Common injected travel vaccinations include MMR (measles, mumps, and rubella), Tdab (tetanus, diphtheria, and pertussis), yellow fever, whereas oral vaccines are administered for typhoid and cholera. Side effects of these travel vaccinations are frequently reported [
8]. These can be either local, i.e., at the site of injection, or systemic, i.e., throughout the body. The US Centers for Disease Control and Prevention (CDC) reports that common local side effects of travel vaccination include pain at the injection site, redness, or swelling, whereas common systemic side effects may include mild fever, headache, feeling tired, nausea, vomiting, diarrhea, and stomach pain [
8]. However, the presence and severity of side effects may differ between vaccination, and it cannot be predicted beforehand which side effects will be experienced, and to what extent.
The risk of experiencing side effects is the most frequently reported reason of fear against vaccination [
9], and the most important reason to refuse vaccinations [
10]. The necessity of vaccination may be a reason for some travelers to reconsider their travel plans, whereas others may decide to go on holiday unprotected by not acquiring the vaccinations.
An effective and safe treatment of possible side effects of vaccinations could help to increase the willingness to vaccinate. Although at present such medicines are not available, SJP-003, the combination of a nonsteroid anti-inflammatory drug, NSAID (naproxen) and an antihistamine drug (fexofenadine), is being developed for this purpose. Here, we describe a case study of an individual that used the combination of naproxen and fexofenadine when receiving multiple travel vaccines.
2. Methods
This case study comprises a 56-year-old male employee of Sen-Jam Pharmaceutical. He was aware that SJP-003 was under development, and that its constituents, naproxen and fexofenadine, are marketed as over-the-counter (OTC) drugs in the USA. The individual volunteered to use the drug combination, as he had scheduled several travel vaccinations for a planned trip to Africa.
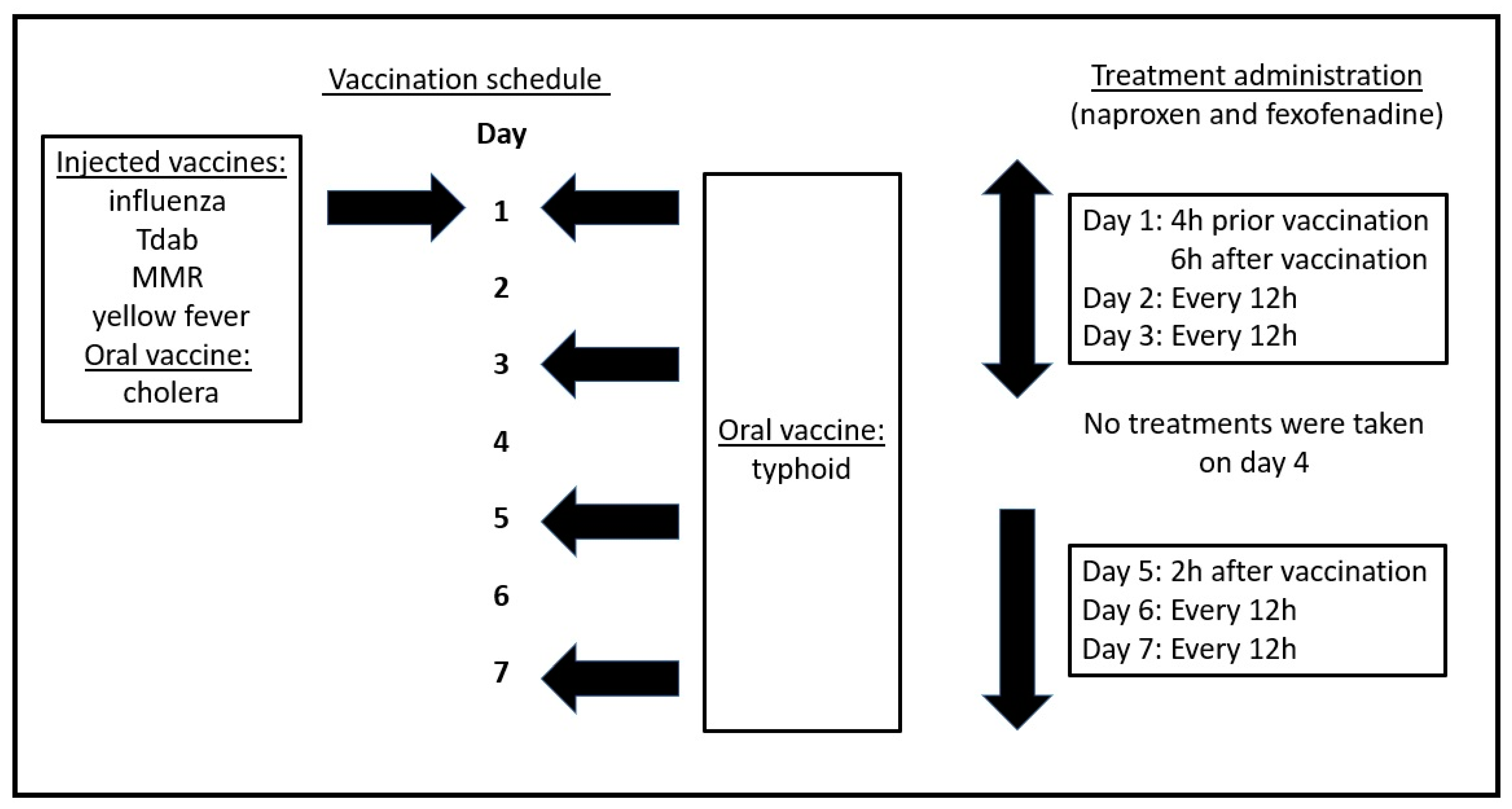
On day 1, he received 2 intramuscular injections. One vaccine was against influenza (Fluzone® quadrivalent by Sanofi Pasteur, Lyon, France), whereas the other vaccine was a booster (Boostrix®, GlaxoSmithKline Biologicals, Brentford, UK) against Tdab (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis). In addition, he received one subcutaneous injection against MMR (measles, mumps, and rubella) and another subcutaneous injection against yellow fever (Stamaril®, Sanofi Pasteur). On day 1, he further received an oral cholera vaccine (100cc, Vaxchora®, Emergent Travel Health, Inc., Redwood, CA, USA). Finally, he also self-administered an oral vaccine against typhoid (Vivotif®, Berna Biotech, Ltd., Bern, Switzerland) on days 1, 3, 5 and 7.
He self-administered a first dose of SJP-003 (220 mg of naproxen sodium and 180 mg of fexofenadine HCL) four hours before vaccinations on day 1. A second dosing was taken 6 h after the vaccinations on day 1, and every 12 h thereafter until the end of day 7. In case no side effects were experienced, treatment could be discontinued. Since the individual self-administered the medication, treatment was not blinded. No ethics approval was needed for the self-administration of these OTC drugs.
The individual described to the corresponding author his experience of side effects temporally associated with vaccination. This was conducted via phone and text messages. The severity of the side effects of the vaccinations were rated on a scale ranging from 0 (absent) to 10 (extreme), and included flu-like symptoms (fever, headache, chills, and muscle aches) and pain at the injection site. The side effects were rated daily, 2 h and 10 h after vaccination.
3. Results
This case was a 56-year-old male individual. He was healthy and had no underlying disease (by self-report). He obtained his travel vaccinations, and an overview of his actual treatment schedule with SJP-003 is given in
Figure 1.
He self-administered the first dose of SJP-003 4 h prior to the vaccinations on day 1. He reported a slight bruising at the injection site which lasted until the end of day 2. However, no injection site pain or flu-like symptoms were experienced. A second dose of SJP-003 was taken 6 h after the vaccinations on day 1, and every 12 h thereafter. On day 3, 12 h thereafter, he self-administered his second oral dose of the typhoid vaccine. No flu-like symptoms or other side effects were reported. After day 3, no side effects of the vaccination were experienced, and he discontinued the treatment. On day 5, two hours after self-administering the third oral dose of the typhoid vaccine, he experienced several flu-like symptoms. An overview of all reported symptoms is given in
Table 1.
At 2 h after vaccination, he self-administered a single dose of SJP-003. The reported adverse reactions were resolved within 4 h. He continued self-administering SJP-003 every 12 h, through day 7. No side effects were reported on day 6. Additionally, after self-administering the final dose of the oral typhoid vaccine on day 7, no side effects of the vaccine were reported.
4. Discussion
The chances of experiencing undesirable side effects from travel vaccinations must be considered as high. For example, the package insert of the oral Vaxchora
® cholera vaccine [
11] reports on a study among 2789 US and Australian adults, 18 to 45 years old, that the most frequently reported side effects over a 7-day period were tiredness (31.3%), headache (28.9%), abdominal pain (18.7%), nausea and/or vomiting (18.3%), and lack of appetite (16.5%). For the Boostrix
® Tdab subcutaneously injected vaccination, the package insert reports on a study among 1480 19-to-65-year-old individuals [
12] which also reported high percentages of side effects, including pain (61%), redness (21%), and swelling (18%) at the site of injection, and systemic effects such as headache (30%), fatigue (28%), gastrointestinal symptoms (16%), and fever (6%). These high percentages of side effects do not contribute to the willingness among the general public to get vaccinated. The recommended solution by the Immunization Action Coalition (IAC) to manage and reduce these side effects, i.e., to apply a cold compress to the injection site or consider an OTC pain reliever [
13]. It is unlikely that this advice is sufficiently reassuring to convince people that hesitate about getting vaccinated to do so. Instead, it is believed that the availability of an effective and safe medicine that prevents side effects from occurring will be of significantly more use to increase willingness to vaccinate.
This case report illustrates the fact that usually multiple travel vaccines are administered in combination This common practice increases the likelihood of experiencing undesirable side effects, and probably also their severity and duration. This case report suggests that SJP-003 was effective in preventing and reducing side effects of travel vaccinations. The side effects of vaccination on Day 5, although of considerable severity, were resolved within 4 h after taking SJP-003.
Naproxen is a commonly used OTC drug for pain relief and its anti-inflammatory properties are well documented [
14]. Fexofenadine is an H1-antagonist with antihistaminergic properties [
15]. As such, it was expected that fexofenadine would reduce vaccination side effects such as rash and itch. In addition, anti-inflammatory properties have also been demonstrated for fexofenadine [
16]. Given the different mechanisms and different activity profiles of the two drugs, the administration of both naproxen and fexofenadine (SJP-003) may have an additive or synergistic effect in preventing or reducing common vaccination side effects such as pain and flu-like symptoms.
However, it must be stressed that this is only a single case study, and all data were self-reported. Therefore, the efficacy of SJP-003 should be confirmed by double-blind, randomized, placebo-controlled clinical trials. These studies should also confirm that SJP-003 has no clinically relevant impact of vaccine efficacy.
Author Contributions
Conceptualization, J.M.I.; methodology, J.M.I.; interpretation of data, P.K., J.C.V., A.S. and J.M.I.; writing—original draft preparation, P.K. and J.C.V.; writing—review and editing, P.K., J.C.V., A.S. and J.M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All available data are presented in this publication.
Conflicts of Interest
Over the past 36 months, A.S. has held research grants from Abbott Nutrition, Arla Foods, Bayer, BioRevive, DuPont, Fonterra, Kemin Foods, Nestlé, Nutricia-Danone, Verdure Sciences. He has acted as a consultant/expert advisor to Arepa Nootroptics, Bayer, Coca-Cola, Danone, Naturex, Nestlé, Pfizer, Sanofi, Sen-Jam Pharmaceutical, and has received travel/hospitality/speaker fees from Bayer, Sanofi, and Verdure Sciences. Over the past 36 months, J.C.V. has acted as a consultant/expert advisor to KNMP, Mentis, Red Bull, Sen-Jam Pharmaceutical, and Toast. J.M.I. is founder and Head of Clinical Development of Sen-Jam Pharmaceutical. P.K. has nothing to declare.
References
- Burns, J.; Movsisyan, A.; Stratil, J.M.; Biallas, R.L.; Coenen, M.; Emmert-Fees, K.M.; Geffert, K.; Hoffmann, S.; Horstick, O.; Laxy, M.; et al. International travel-related control measures to contain the COVID-19 pandemic: A rapid review. Cochrane Database Syst. Rev. 2021, 3, CD013717. [Google Scholar] [PubMed]
- Bou-Karroum, L.; Khabsa, J.; Jabbour, M.; Hilal, N.; Haidar, Z.; Khalil, P.A.; Khalek, R.A.; Assaf, J.; Haidar, G.H.-A.; Samra, C.A.; et al. Public health effects of travel-related policies on the COVID-19 pandemic: A mixed-methods systematic review. J. Infect. 2021, 83, 413–423. [Google Scholar] [PubMed]
- Cretu, R.C.; Stefan, P.; Alecu, I.I. Has tourism gone on holiday? Analysis of the effects of the COVID-19 pandemic on tourism and post-pandemic tourism behavior. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 191–197. [Google Scholar]
- Number of Visitors to Africa from the U.S. from 2015 to 2019, by Month. Available online: https://www.statista.com/statistics/790448/us-citizen-monthly-travel-to-africa/ (accessed on 1 June 2022).
- Pavli, A.; Maltezou, H.C. Travel vaccines throughout history. Travel Med. Infect. Dis. 2022, 46, 102278. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W.A.; Ahmed, R. Simply put: Vaccination saves lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Need Travel Vaccines? Plan Ahead. Available online: https://wwwnc.cdc.gov/travel/page/travel-vaccines (accessed on 1 June 2022).
- Centers for Disease Control and Prevention. Possible Side Effects from Vaccines. Available online: https://www.cdc.gov/vaccines/vac-gen/side-effects.htm (accessed on 1 June 2022).
- Moving the Needle: Promoting Vaccination Uptake Across the Life Course. Available online: https://www.rsph.org.uk/our-work/policy/vaccinations/moving-the-needle-promoting-vaccination-uptake-across-the-life-course.html (accessed on 1 June 2022).
- Why Won’t Americans Get Vaccinated? Available online: https://today.yougov.com/topics/politics/articles-reports/2021/07/15/why-wont-americans-get-vaccinated-poll-data (accessed on 1 June 2022).
- Package Insert Vaxchora. Available online: https://www.fda.gov/media/128415/download (accessed on 1 June 2022).
- Package Insert Boostrix. Available online: https://www.fda.gov/media/124002/download (accessed on 1 June 2022).
- Medical Management of Vaccine Reactions in Adults in a Community Setting. Available online: https://www.immunize.org/catg.d/p3082.pdf (accessed on 1 June 2022).
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Naproxen up to date: A review of its pharmacological properties and therapeutic effcacy and use in rheumatic diseases and pain states. Drugs 1979, 18, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Amichai, B.; Grunwald, M.H.; Brenner, L. Fexofenadine hydrochloride—A new anti-histaminic drug. IMAJ 2001, 3, 207–209. [Google Scholar] [PubMed]
- Ashenager, M.S.; Grgela, T.; Aragane, Y.; Kawada, A. Inhibition of cytokine-induced expression of T-cell cytokines by antihistamines. J. Investig. Allergol. Clin. Immunol. 2007, 17, 20–26. [Google Scholar] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).