Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
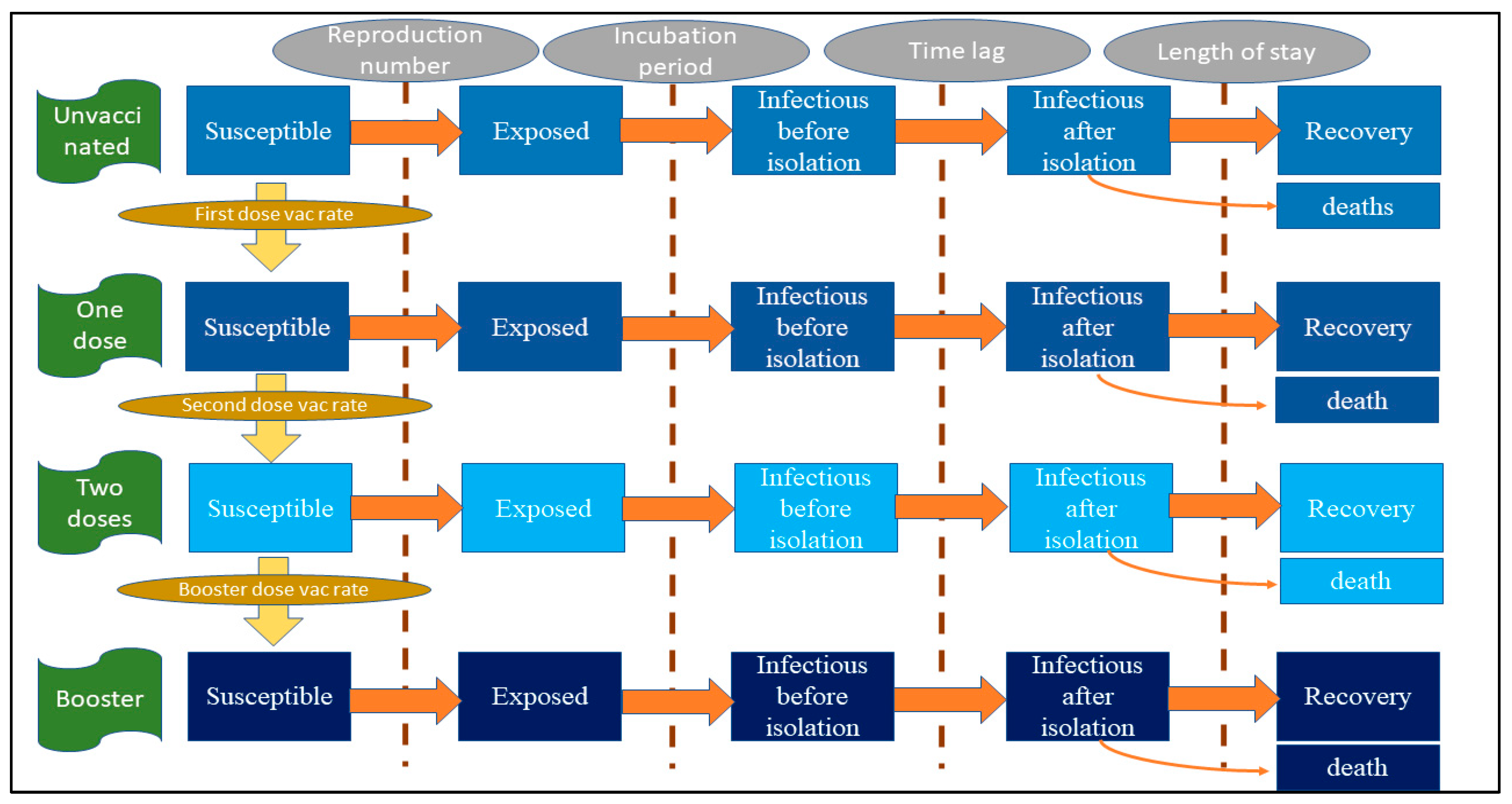
2.2. Model Framework
2.3. Model Assumptions, Parameters and the Formula
2.4. Model Scenarios and Interested Outcomes
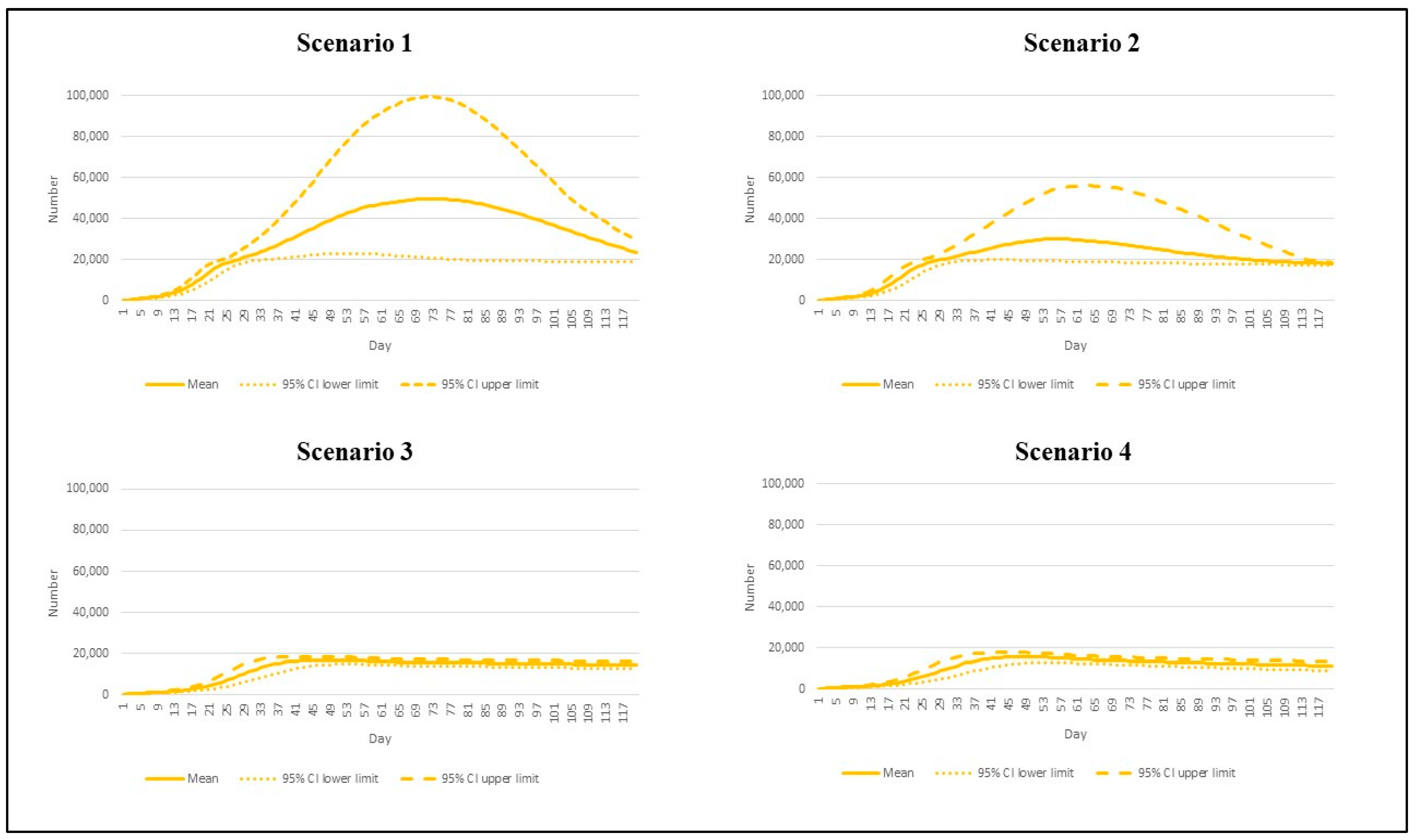
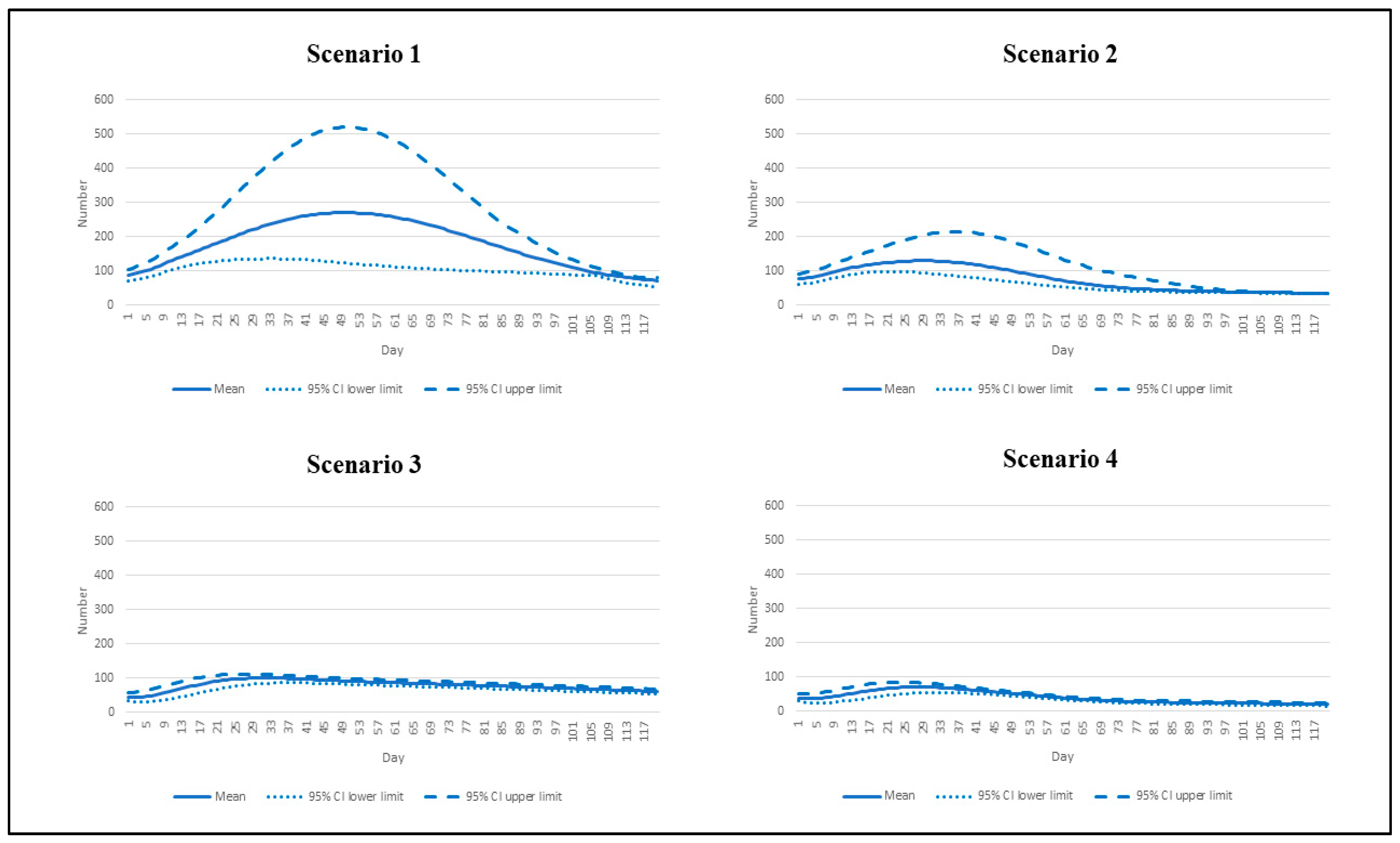
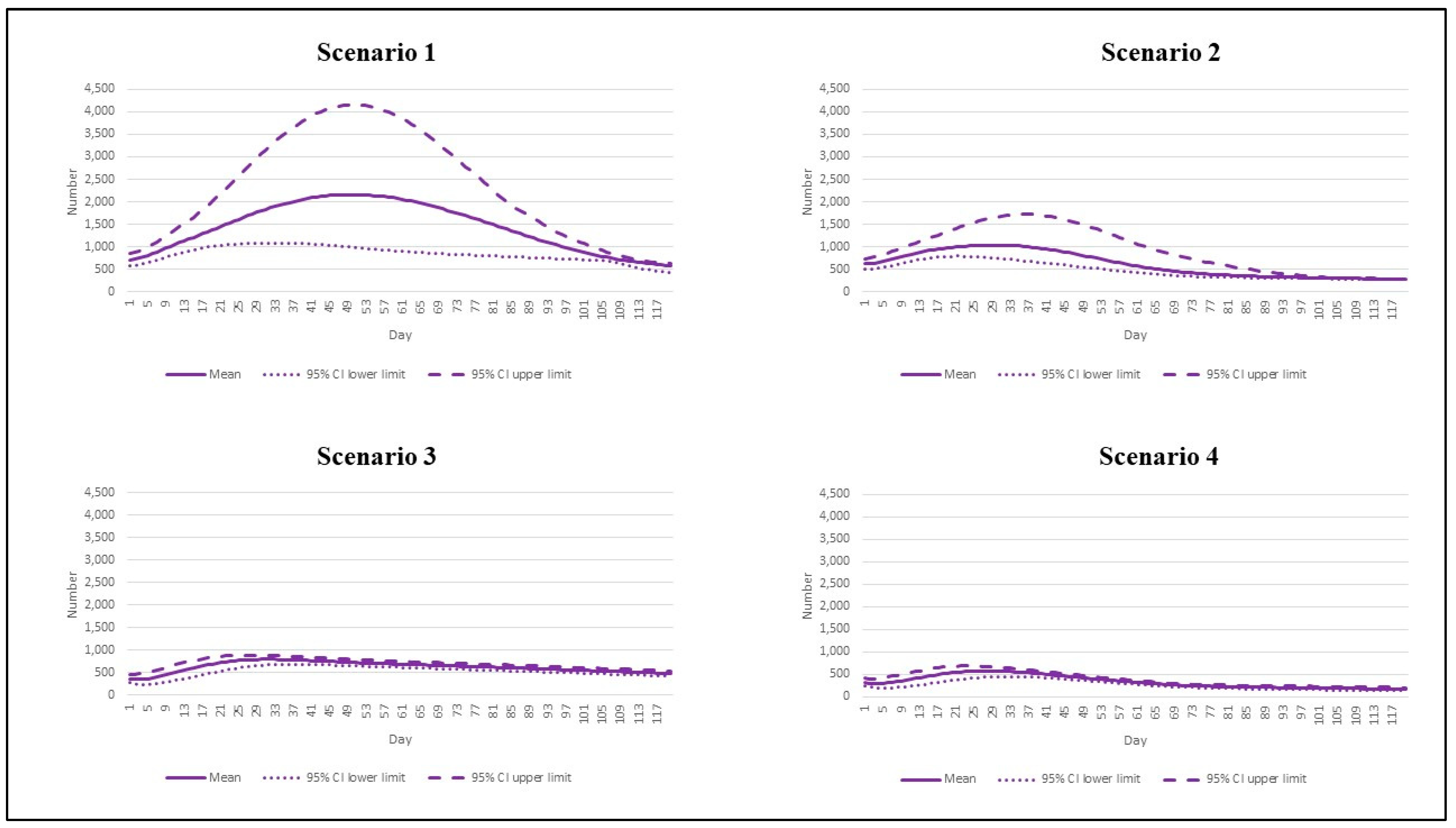
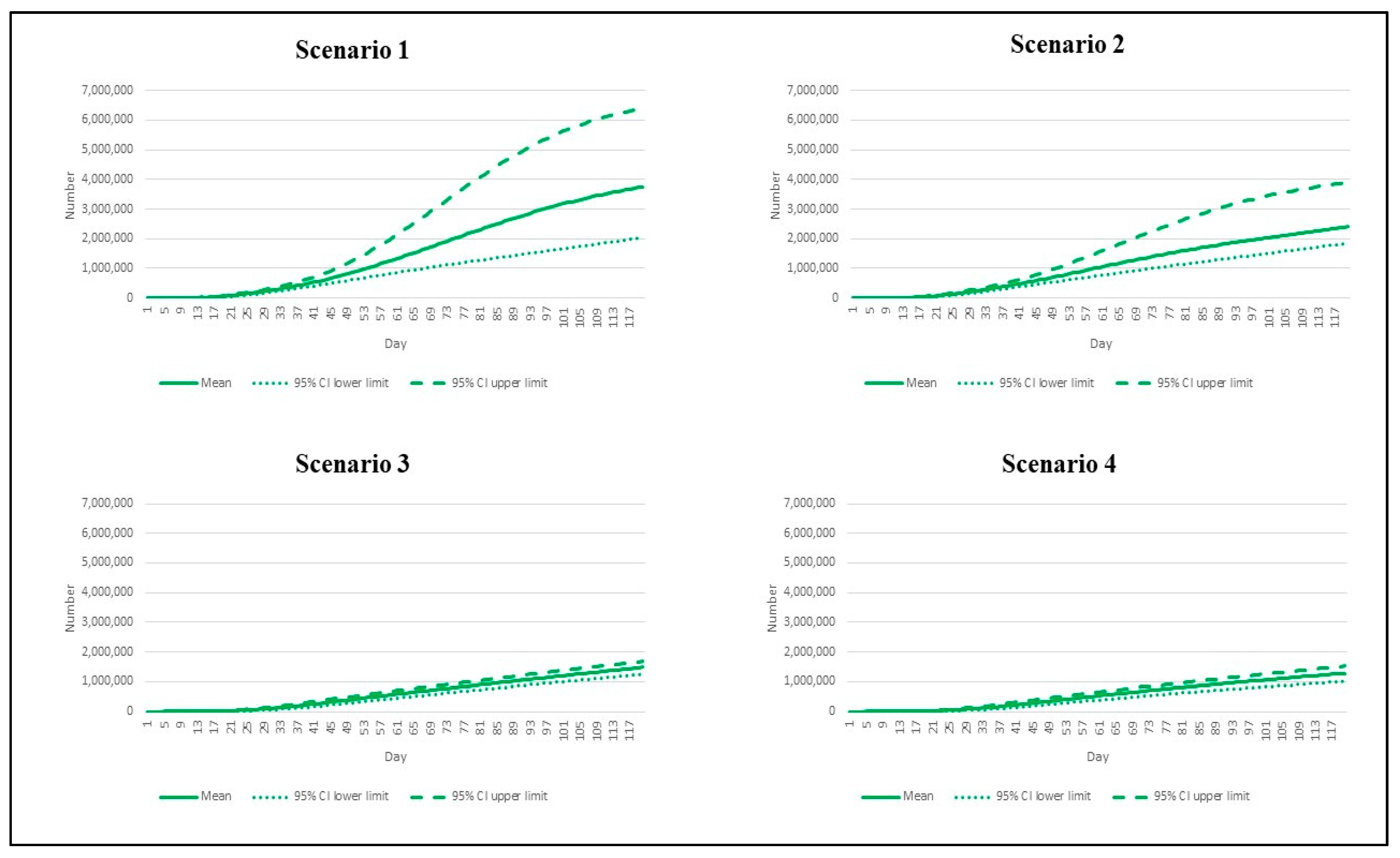
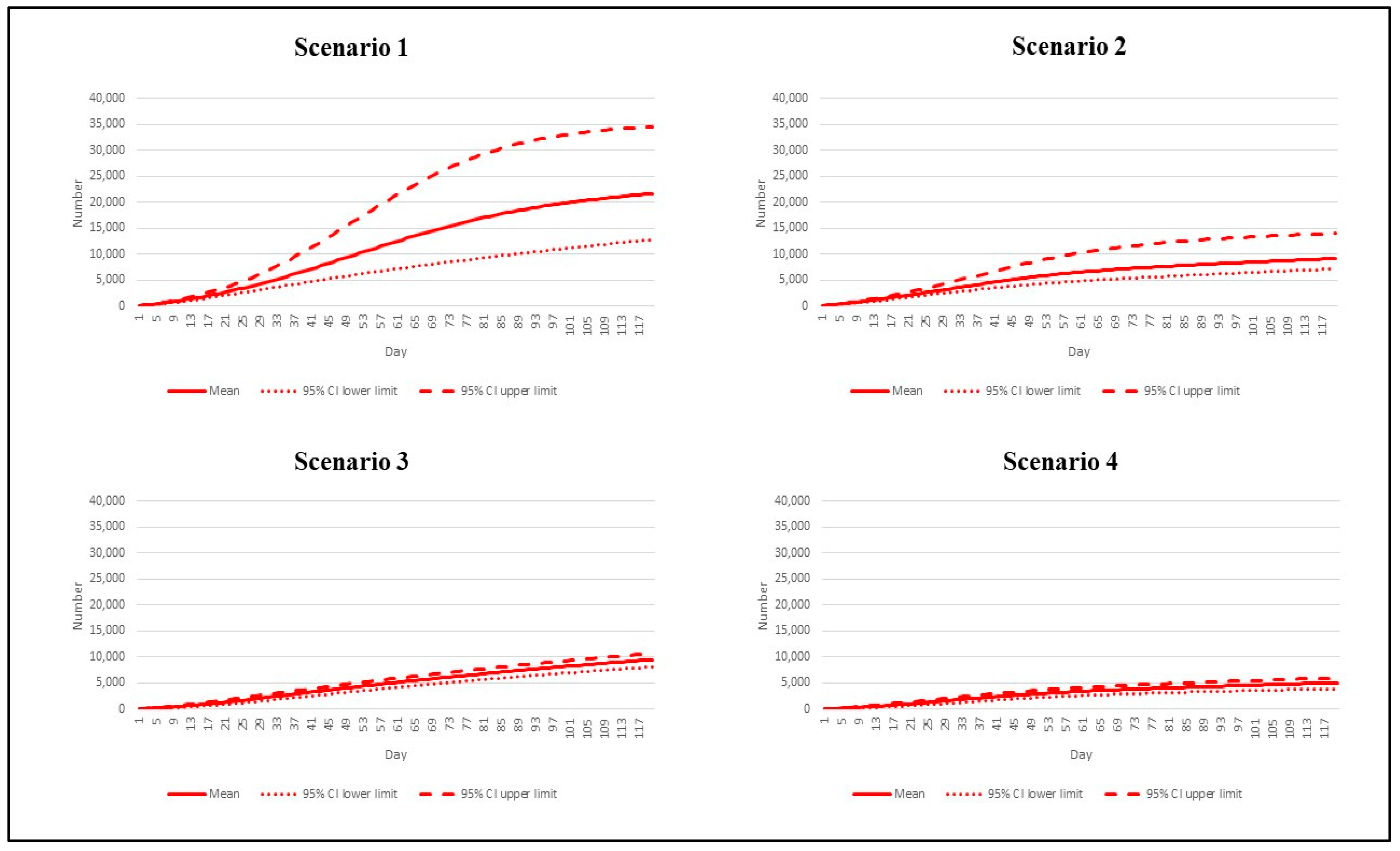
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19); WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 23 December 2021).
- Mohamadi, M.; Babington-Ashaye, A.; Lefort, A.; Flahault, A. Risks of Infection with SARS-CoV-2 Due to Contaminated Surfaces: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 11019. [Google Scholar] [CrossRef] [PubMed]
- Wuhan Municipal Health Commission. Report on Current Pneumonia Epidemic Situation in the City. 2019. Available online: http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 (accessed on 23 December 2021). (In Chinese)
- World Health Organization. Weekly Epidemiological Update on COVID-19—21 December 2021; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-december-2021 (accessed on 23 December 2021).
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tracking SARS-CoV-2 Variants; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 3 January 2022).
- Chookajorn, T.; Kochakarn, T.; Wilasang, C.; Kotanan, N.; Modchang, C. Southeast Asia is an emerging hotspot for COVID-19. Nat. Med. 2021, 27, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Colona, V.L.; Pandolfi, P.P. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J. Med. Res. 2021, 153, 537–541. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J. Med. Virol. 2021, 94, 1821–1824. [Google Scholar] [CrossRef]
- Rajatanavin, N.; Tuangratananon, T.; Suphanchaimat, R.; Tangcharoensathien, V. Responding to the COVID-19 second wave in Thailand by diversifying and adapting lessons from the first wave. BMJ Glob. Health 2021, 6, e006178. [Google Scholar] [CrossRef]
- Uansri, S.; Tuangratananon, T.; Phaiyarom, M.; Rajatanavin, N.; Suphanchaimat, R.; Jaruwanno, W. Predicted Impact of the Lockdown Measure in Response to Coronavirus Disease 2019 (COVID-19) in Greater Bangkok, Thailand, 2021. Int. J. Environ. Res. Public Health 2021, 18, 12816. [Google Scholar] [CrossRef]
- Reuters. Thailand Aims to Vaccinate 70% of People by September; Reuters: Bangkok, Thailand, 2021; Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/thailand-aims-vaccinate-70-people-by-september-2021-05-19/ (accessed on 3 January 2022).
- Dietz, K. The estimation of the basic reproduction number for infectious diseases. Stat. Methods Med. Res. 1993, 2, 23–41. [Google Scholar] [CrossRef]
- Davahli, M.R.; Karwowski, W.; Taiar, R. A system dynamics simulation applied to healthcare: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5741. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Murthy, B.S.N.; Samad, S.A.; Biswas, M.H.A. Mathematical transmission analysis of SEIR tuberculosis disease model. Sens. Int. 2021, 2, 100120. [Google Scholar] [CrossRef]
- Tan, X.; Yuan, L.; Zhou, J.; Zheng, Y.; Yang, F. Modeling the initial transmission dynamics of influenza A H1N1 in Guangdong Province, China. Int. J. Infect. Dis. 2013, 17, e479-e84. [Google Scholar] [CrossRef][Green Version]
- Wang, J.-j.; Reilly, K.H.; Luo, J.; Zang, C.-p.; Wang, N. Dynamic mathematical models of HIV/AIDS transmission in China. Chin. Med. J. 2010, 123, 2120–2127. [Google Scholar] [PubMed]
- Department of Disease Control Ministry of Public Health Thailand. Corona Virus Disease (COVID-19): Thailand Situation. 2021. Available online: https://ddc.moph.go.th/viralpneumonia/eng/index.php (accessed on 26 August 2021).
- National Health Security Office. NHSO Launches Supported Home and Community Isolation, Ramps up Rapid COVID-19 Tests; NHSO: Bangkok, Thailand, 2011. Available online: https://eng.nhso.go.th/view/1/DescriptionNews/NHSO-launches-supported-home-and-community-isolation-ramps-up-rapid-COVID-19-tests/354/EN-US (accessed on 5 January 2022).
- Zhang, X.; Wu, S.; Wu, B.; Yang, Q.; Chen, A.; Li, Y.; Zhang, Y.; Pan, T.; Zhang, H.; He, X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct. Target. Ther. 2021, 6, 430. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Brauer, F. Compartmental Models in Epidemiology. In Mathematical Epidemiology; Brauer, F., van den Driessche, P., Wu, J., Eds.; Lecture Notes in Mathematic; Springer: Berlin/Heidelberg, Germany, 2008; Volume 1945. [Google Scholar]
- Ito, K.; Piantham, C.; Nishiura, H. Relative Instantaneous Reproduction Number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J. Med. Virol. 2021, 94, 2265–2268. [Google Scholar] [CrossRef]
- Head, E.; van Elsland, S. Omicron Largely Evades Immunity from Past Infection or Two Vaccine Doses; Imperial College: London, UK, 2021; Available online: https://www.imperial.ac.uk/news/232698/omicron-largely-evades-immunity-from-past/ (accessed on 5 January 2022).
- Barnard, R.C.; Davies, N.G.; Pearson, C.A.B.; Jit, M.; Edmunds, W.J. Projected epidemiological consequences of the Omicron SARS-CoV-2 variant in England, December 2021 to April 2022. medRxiv 2021. [Google Scholar] [CrossRef]
- Our World in Data. COVID-19: Google Mobility Trends; Our World in Data: Oxford, UK, 2021; Available online: https://ourworldindata.org/covid-google-mobility-trends (accessed on 6 January 2022).
- National Statistical Office of Thailand. Demographic and Household Statistics; Bangkok Report; 2021. Available online: http://statbbi.nso.go.th/staticreport/page/sector/th/01.aspx (accessed on 15 July 2021).
- Hart, W.S.; Miller, E.; Andrews, N.J.; Waight, P.; Maini, P.K.; Funk, S.; Thompson, R.N. Generation time of the Alpha and Delta SARS-CoV-2 variants. medRxiv 2021. [Google Scholar] [CrossRef]
- Helmsdal, G.; Hansen, O.K.; Møller, L.F.; Christiansen, D.H.; Petersen, M.S.; Kristiansen, M.F. Omicron outbreak at a private gathering in the Faroe Islands, infecting 21 of 33 triple-vaccinated healthcare workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Caspani, M.; Stapleton, S. Omicron Surge Pushes U.S. COVID Hospitalizations toward Record High; Reuters: London, UK, 2022; Available online: https://www.reuters.com/world/us/omicron-pushes-us-covid-hospitalizations-toward-record-high-2022-01-07/ (accessed on 8 January 2022).
- UK Health Security Agency. Weekly National Influenza and COVID-19 Surveillance Report: Week 1 Report (Up to Week 52 Data) 6 January 2022; UK Health Security Agency: London, UK, 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045027/Weekly_Flu_and_COVID-19_report_w1.pdf (accessed on 8 January 2022).
- BBC News. South Africa Says Omicron Wave May Have Peaked; BBC: London, UK, 2022; Available online: https://www.bbc.com/news/world-africa-59832843 (accessed on 8 January 2022).
- Aljazeera. Hospitals in Thailand Short of Beds as COVID Cases Soar: Ministry; Aljazeera: Bangkok, Thailand, 2021; Available online: https://www.aljazeera.com/news/2021/7/29/hospitals-in-thailand-short-of-beds-as-covid-cases-soar-ministry (accessed on 9 January 2022).
- Barach, P.; Fisher, S.D.; Adams, M.J.; Burstein, G.R.; Brophy, P.D.; Kuo, D.Z.; Lipshultz, S.E. Disruption of healthcare: Will the COVID pandemic worsen non-COVID outcomes and disease outbreaks? Prog. Pediatr. Cardiol. 2020, 59, 101254. [Google Scholar] [CrossRef] [PubMed]
- Clerk, A.M. Beware of Neglect of Non-COVID Patients in COVID Era. Indian J. Crit. Care Med. 2021, 25, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Effectiveness of COVID-19 vaccines against Omicron or Delta infection. medRxiv 2022. [Google Scholar] [CrossRef]
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing: Update on Hospitalisation and Vaccine Effectiveness for Omicron VOC-21NOV-01 (B.1.1.529); UK Health Security Agency: London, UK, 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1044481/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf (accessed on 9 January 2022).
- Wipatayotin, A. New Drive to Give 1 m Doses per Day; Bangkok Post: Bangkok, Thailand, 2021; Available online: https://www.bangkokpost.com/thailand/general/2187275/new-drive-to-give-1m-doses-per-day (accessed on 9 January 2022).
- Phan, T.; Boes, S.; McCullough, M.; Gribschaw, J.; Marsh, J.W.; Harrison, L.H.; Wells, A. First detection of SARS-CoV-2 Omicron BA.4 variant in Western Pennsylvania, United States. J. Med. Virol. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Unit | Value | Reference (Note) |
|---|---|---|---|
| Reproduction number | Unitless | 4.3–7.5 | Ito et al. [25], Head and van Elsland [26] (3.1–5.4 times greater than the Delta epidemic in Thailand in 2020) |
| Population | Persons | 66.2 × 106 | National Statistical Office of Thailand [29] (assume homogenous mixing) |
| Mean infectious duration | Days | 4.6 | Hart et al. [30] (assume gamma distribution with scale parameter of 0.03 and shape parameter of 165.9—same as the Delta variant) |
| Mean incubation period | Days | 3.2 | Helmsdal et al. [31] (assume gamma distribution with scale parameter of 0.01 and shape parameter of 302.7—shorter than the Delta variant) |
| Time lag from being infected to isolation | Days | 5 | Model calibration (assume same as the Delta epidemic in Thailand in 2020) |
| Initial number of infectees | Persons | 10,000 | Model calibration (assume fourfold greater than the present incident cases) |
| Initial proportion of unvaccinated population | Unitless | 0.26 | Internal database of the Department of Disease Control |
| Initial proportion of one-dose vaccinees | Unitless | 0.09 | Internal database of the Department of Disease Control |
| Initial proportion of two-dose vaccinees | Unitless | 0.58 | Internal database of the Department of Disease Control |
| Initial proportion booster-dose vaccinees | Unitless | 0.06 | Internal database of the Department of Disease Control |
| First-dose vaccination base rate | Persons/day | 66,200 | Internal database of the Department of Disease Control (assume equaling the rate of booster dose) |
| Second-dose vaccination base rate | Persons/day | 198,600 | Internal database of the Department of Disease Control (the largest rate compared to other doses as the second shot currently being the main policy priority) |
| Booster-dose vaccination base rate | Persons/day | 66,200 | Internal database of the Department of Disease Control |
| Vaccine effectiveness against any infection for one-dose vaccination | Unitless | 0.17 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Vaccine effectiveness against any infection for two-dose vaccination | Unitless | 0.41 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Vaccine effectiveness against any infection for booster-dose vaccination | Unitless | 0.65 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Vaccine effectiveness against severe infection for one-dose vaccination | Unitless | 0.70 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Vaccine effectiveness against severe infection for two-dose vaccination | Unitless | 0.90 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Vaccine effectiveness against severe infection for booster-dose vaccination | Unitless | 0.95 | Adapted from Head and van Elsland [26] and internal database of the Department of Disease Control |
| Proportion of intubated cases to existing active infectees | Unitless | 0.005 | Internal database of the Department of Disease Control (assume half of the proportion of the Delta variant) |
| Ratio of deaths per existing intubated cases | Unitless | 0.03 | Internal database of the Department of Disease Control (assume same as the ratio of the Delta variant) |
| Length of stay for non-intubated cases | Days | 10 | Internal database of the Department of Disease Control |
| Length of stay for intubated cases | Days | 21 | Internal database of the Department of Disease Control |
| Non-pharmaceutical intervention base effectiveness against any infection | Unitless | See supporting information (Figure S1) | Assume being an exponential function with the incident cases |
| Description | Formula | Note |
|---|---|---|
| Rate of change from being susceptible to being exposed | −β × (1 − κ) × (1 − ve) × S × I1/P | β = reproduction number/infectious duration, κ = effectiveness of non-pharmaceutical intervention against any infection, ve = effectiveness of vaccine against any infection (varying by vaccine doses), S = susceptible population, I1 = non-isolated infectees, P = total population |
| Rate of change from being susceptible to being non-isolated infectious | −αE | α = 1/incubation period, E = exposed population |
| Rate of change from being non-isolated infectious to being isolated infectious | −δI1 | δ = 1/time lag from non-isolation to isolation, I1 = non-isolated infectious population |
| Rate of change from being isolated infectious to being recovered | −ζI2 | ζ = 1/length of stay; I2 = isolated infectious population (varying by severity status) |
| Scenario | Reproduction Number | Vaccination Rate |
|---|---|---|
| 1 | 7.5 | Base pace |
| 2 | 7.5 | Speedy pace |
| 3 | 4.3 | Base pace |
| 4 | 4.3 | Speedy pace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suphanchaimat, R.; Teekasap, P.; Nittayasoot, N.; Phaiyarom, M.; Cetthakrikul, N. Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022. Vaccines 2022, 10, 1024. https://doi.org/10.3390/vaccines10071024
Suphanchaimat R, Teekasap P, Nittayasoot N, Phaiyarom M, Cetthakrikul N. Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022. Vaccines. 2022; 10(7):1024. https://doi.org/10.3390/vaccines10071024
Chicago/Turabian StyleSuphanchaimat, Rapeepong, Pard Teekasap, Natthaprang Nittayasoot, Mathudara Phaiyarom, and Nisachol Cetthakrikul. 2022. "Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022" Vaccines 10, no. 7: 1024. https://doi.org/10.3390/vaccines10071024
APA StyleSuphanchaimat, R., Teekasap, P., Nittayasoot, N., Phaiyarom, M., & Cetthakrikul, N. (2022). Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022. Vaccines, 10(7), 1024. https://doi.org/10.3390/vaccines10071024







