Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- non-allergic reaction to non-polysorbate-containing vaccines (e.g., expected side effects, subjective symptoms or local injection site reactions)
- (2)
- previously tolerated polysorbate-containing vaccines, or
- (3)
- no allergies to non-COVID vaccines.
Statistical Analysis
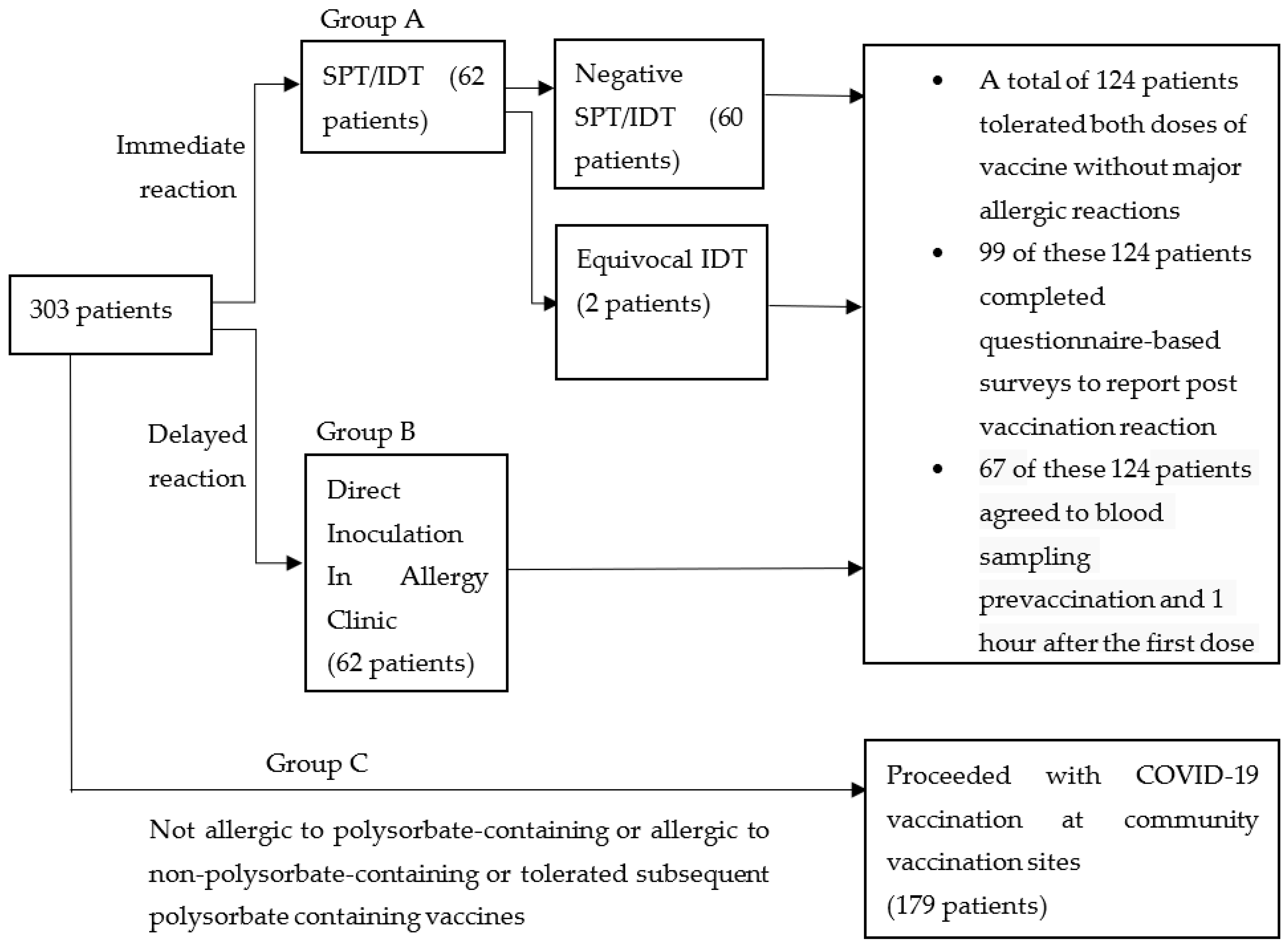
3. Results
Group A (SPT/IDT) and Group B (DI) Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kelso, J.M. Anaphylactic reactions to novel mRNA SARS-CoV-2/COVID-19 vaccines. Vaccine 2021, 39, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Health Sciences Authority. Singapore Interim Authorisation of Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) for Active Immunisation to Prevent COVID-19 Disease in Singapore. Available online: https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/interim-authorisation-of-pfizer-biontech-covid-19-vaccine-(bnt162b2)-for-active-immunisation-to-prevent-covid-19-disease-in-singapore (accessed on 14 December 2020).
- Health Sciences Authority. Singapore HSA Grants Interim Authorisation for Moderna COVID-19 Vaccine in Singapore. Available online: https://www.hsa.gov.sg/announcements/press-release/hsa-grants-interim-authorisation-for-moderna-covid-19-vaccine-in-singapore (accessed on 3 February 2021).
- Rüggeberg, J.U.; Gold, M.S.; Bayas, J.-M.; Blum, M.D.; Bonhoeffer, J.; Friedlander, S.; Brito, G.D.S.; Heininger, U.; Imoukhuede, B.; Khamesipour, A.; et al. Brighton Collaboration Anaphylaxis Working Group. Anaphylaxis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007, 25, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.R.; Leung, B.P.; Ng, C.Y.L.; Tan, J.W.L.; Chan, G.Y.L.; Loh, C.M.; Tan, G.L.X.; Goh, V.H.H.; Wong, L.T.; Chua, C.R.; et al. Pseudo-Anaphylactic Reactions to Pfizer BNT162b2 Vaccine: Report of 3 Cases of Anaphylaxis Post Pfizer BNT162b2 Vaccination. Vaccines 2021, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Risma, K.A.; Edwards, K.M.; Hummell, D.S.; Little, F.F.; Norton, A.E.; Stallings, A.; Wood, R.A.; Milner, J. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J. Allergy Clin. Immunol. 2021, 147, 2075–2082.e2. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.; Shaker, M.; Golden, D.B.K. PEG/Polysorbate Skin Testing Has No Utility in the Assessment of Suspected Allergic Reactions to SARS-CoV-2 Vaccines. J. Allergy Clin. Immunol Pract. 2021, 9, 3321–3322. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, M.M.; Sitek, A.N.; Kinate, S.A.; Joshi, A.Y.; Park, M.A. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: Early report. Ann. Allergy Asthma Immunol. 2021, 126, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, M.M.; Sitek, A.N.; D’Netto, M.E.; Dages, K.N.; Chiarella, S.E.; Gonzalez-Estrada, A.; Joshi, A.Y.; Park, M.A. Utility and futility of skin testing to address concerns surrounding messenger RNA coronavirus disease 2019 vaccine reactions. Ann. Allergy Asthma Immunol. 2022, 128, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.R.; Robinson, L.B.; Li, L.; McMahon, A.E.; Cogan, A.S.; Fu, X.; Wickner, P.; Samarakoon, U.; Saff, R.R.; Blumenthal, K.G.; et al. First-Dose mRNA COVID-19 Vaccine Allergic Reactions: Limited Role for Excipient Skin Testing. J. Allergy Clin. Immunol. Pract. 2021, 9, 3308–3320.e3. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, Z.; Wei, X.; Shi, J.; Li, C. Antibodies against polyethylene glycol in human blood: A literature review. J. Pharmacol. Toxicol. Methods 2020, 102, 106678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal. Chem. 2016, 88, 1804–11812. [Google Scholar] [CrossRef] [PubMed]
- Troelnikov, A.; Perkins, G.; Yuson, C.; Ahamdie, A.; Balouch, S.; Hurtado, P.R.; Hissaria, P. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J. Allergy Clin. Immunol. 2021, 148, 91–95. [Google Scholar] [CrossRef] [PubMed]
| Demographics | SPT/IDT and DI Cohort n = 124 | Suspected Immediate Hypersensitivity to Polysorbate-Containing Vaccines; SPT/IDT Cohort n = 62 | Suspected Delayed Hypersensitivity to Polysorbate-Containing Vaccines; DI Cohort n = 62 | p-Values |
|---|---|---|---|---|
| Age, mean (SD) | 52.4 (16.9) | 49.8 (16.0) | 54.9 (17.6) | p = 0.091 |
| Sex (female, %) | 82 (66.1%) | 44 (71%) | 38 (61.3%) | p = 0.255 |
| Race (%) | ||||
| Chinese | 88 (71%) | 44 (71%) | 44 (71%) | p = 1.000 |
| Malay | 9 (7.3%) | 5 (8%) | 4 (6.5%) | p = 0.729 |
| Indian | 14 (11.3%) | 6 (9.7%) | 8 (12.9%) | p = 0.570 |
| Others | 13 (10.5%) | 7 (11.3%) | 6 (9.7%) | p = 0.769 |
| Allergic and atopic conditions | ||||
| Allergic rhinitis | 24 (19.4%) | 15 (24.2%) | 9 (14.5%) | p = 0.173 |
| Asthma | 17 (13.7%) | 10 (16.1%) | 7 (11.3%) | p = 0.433 |
| Chronic spontaneous urticarial | 11 (8.9%) | 3 (4.8%) | 8 (12.9%) | p = 0.114 |
| Food allergy | 14 (11.3%) | 6 (9.7%) | 8 (12.9%) | p = 0.570 |
| Eczema | 14 (11.3%) | 7 (11.3%) | 7 (11.3%) | p = 1.000 |
| Drug allergy | 49 (40%) | 25 (40.3%) | 24 (38.7%) | p = 0.854 |
| Vaccine reactions | ||||
| Urticaria only | 26 (21%) | 14 (22.6%) | 12 (19.4%) | p = 0.659 |
| Angioedema only | 17 (13.7%) | 11 (17.7%) | 6 (9.7%) | p = 0.192 |
| Cutaneous | 68 (54.8%) | 36 (58.1%) | 32 (51.6%) | p = 0.470 |
| Upper airway | 3 (2.4%) | 1 (1.6%) | 2 (3.2%) | p = 0.559 |
| Lower airway | 9 (7.3%) | 3 (4.8%) | 6 (9.7%) | p = 0.299 |
| Cardiovascular | 6 (4.8%) | 2 (3.2%) | 4 (6.5%) | p = 0.403 |
| Gastrointestinal | 3 (2.4%) | 1 (1.6%) | 2 (3.2%) | p = 0.559 |
| Anaphylaxis | 1 (0.8%) | 0 (0%) | 1 (1.6%) | p = 1.000 * |
| Unknown | 2 (1.6%) | 0 (0%) | 2 (3.2%) | p = 0.496 * |
| Polysorbate-Containing Vaccines | Number of Patients (%) |
| Influenza | 54 (43.5%) |
| Tetanus | 31 (25.8%) |
| Hepatitis B | 6 (4.8%) |
| Pneumococcal | 10 (8.1%) |
| Human Papilloma Virus | 6 (4.8%) |
| Tetanus, Diphtheria and Pertussis | 8 (6.5%) |
| Varicella | 1 (0.80%) |
| Hepatitis A | 3 (2.4%) |
| Meningococcal | 1 (0.80%) |
| Non-Polysorbate-Containing Vaccines | Number of Patients (%) |
| Bacillus Calmette–Guérin | 3 (2.4%) |
| Polio | 4 (3.2%) |
| Typhoid | 3 (2.4%) |
| Yellow fever | 2 (1.6%) |
| Rabies | 1 (0.80%) |
| Measles, Mumps, Rubella | 7 (5.6%) |
| Laboratory Tests | Reference Ranges | Non-Reactors, n = 58 | Reactors, n = 9 | p-Value | ||
|---|---|---|---|---|---|---|
| Pre-Vaccination | 1 h Post Vaccination | Pre-Vaccination | 1 h Post Vaccination | |||
| Anti-BNT162b2 IgE (ng/mL) | N.A. | <0.45 (below detection limit) | N.A. | <0.45 (below detection limit) | N.A. | N.D. |
| IL-4 (pg/mL) | <2 | <1 (below detection limit) | <1 (below detection limit) | <1 (below detection limit) | <1 (below detection limit) | N.D. |
| IL-33 (pg/mL) | <2 | 1.69 ± 0.95 | 1.52 ± 0.86 | 1.74 ± 0.97 | 1.30 ± 0.26 | 0.925 |
| C5a (ng/mL) | 47.42 ± 31.07 | 51.96 ± 27.10 | 50.88 ± 23.14 | 53.53 ± 30.58 | 48.18 ± 22.47 | 0.903 |
| ICAM-1 (ng/mL) | <95 | 58.80 ± 24.76 | 55.76 ± 25.83 | 53.59 ± 17.04 | 58.35 ± 19.85 | 0.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, X.R.; Tan, J.W.L.; Chan, G.Y.L.; Hou, J.; Xie, L.; Goh, V.H.L.; Boon, J.; Lee, S.S.M.; Teo, C.M.-L.; Tan, S.C.; et al. Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination. Vaccines 2022, 10, 1025. https://doi.org/10.3390/vaccines10071025
Lim XR, Tan JWL, Chan GYL, Hou J, Xie L, Goh VHL, Boon J, Lee SSM, Teo CM-L, Tan SC, et al. Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination. Vaccines. 2022; 10(7):1025. https://doi.org/10.3390/vaccines10071025
Chicago/Turabian StyleLim, Xin Rong, Justina Wei Lynn Tan, Grace Yin Lai Chan, Jinfeng Hou, Linlin Xie, Vivian Hui Li Goh, Joewee Boon, Samuel Shang Ming Lee, Claire Min-Li Teo, Sze Chin Tan, and et al. 2022. "Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination" Vaccines 10, no. 7: 1025. https://doi.org/10.3390/vaccines10071025
APA StyleLim, X. R., Tan, J. W. L., Chan, G. Y. L., Hou, J., Xie, L., Goh, V. H. L., Boon, J., Lee, S. S. M., Teo, C. M.-L., Tan, S. C., Leong, K. P., Thong, B. Y. H., & Leung, B. P. L. (2022). Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination. Vaccines, 10(7), 1025. https://doi.org/10.3390/vaccines10071025






