Effectiveness of the ChAdOx1 nCoV-19 Coronavirus Vaccine (CovishieldTM) in Preventing SARS-CoV2 Infection, Chennai, Tamil Nadu, India, 2021
Abstract
:1. Introduction
2. Materials and Methods
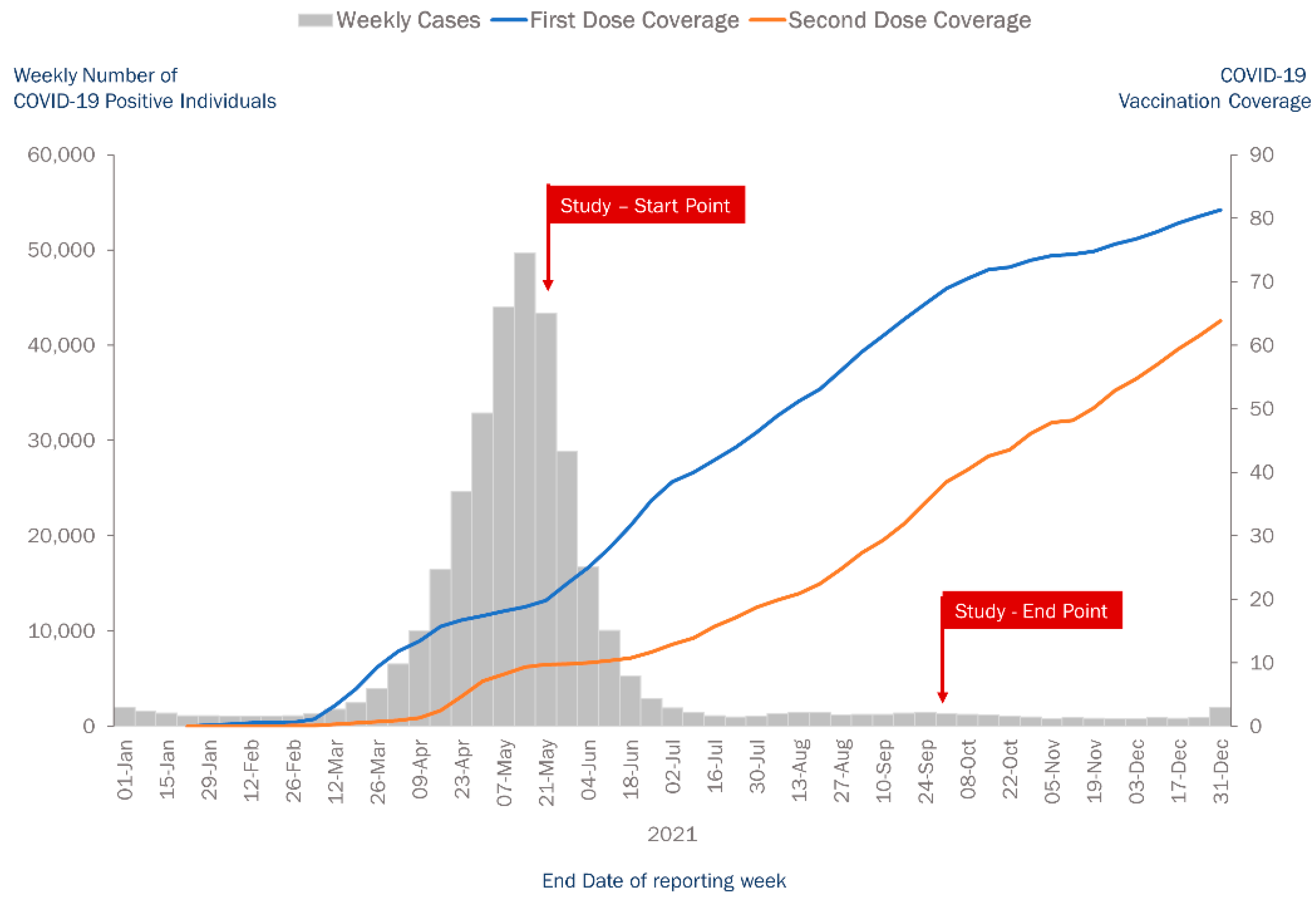
2.1. Study Design and Setting
2.2. Study Population and Period
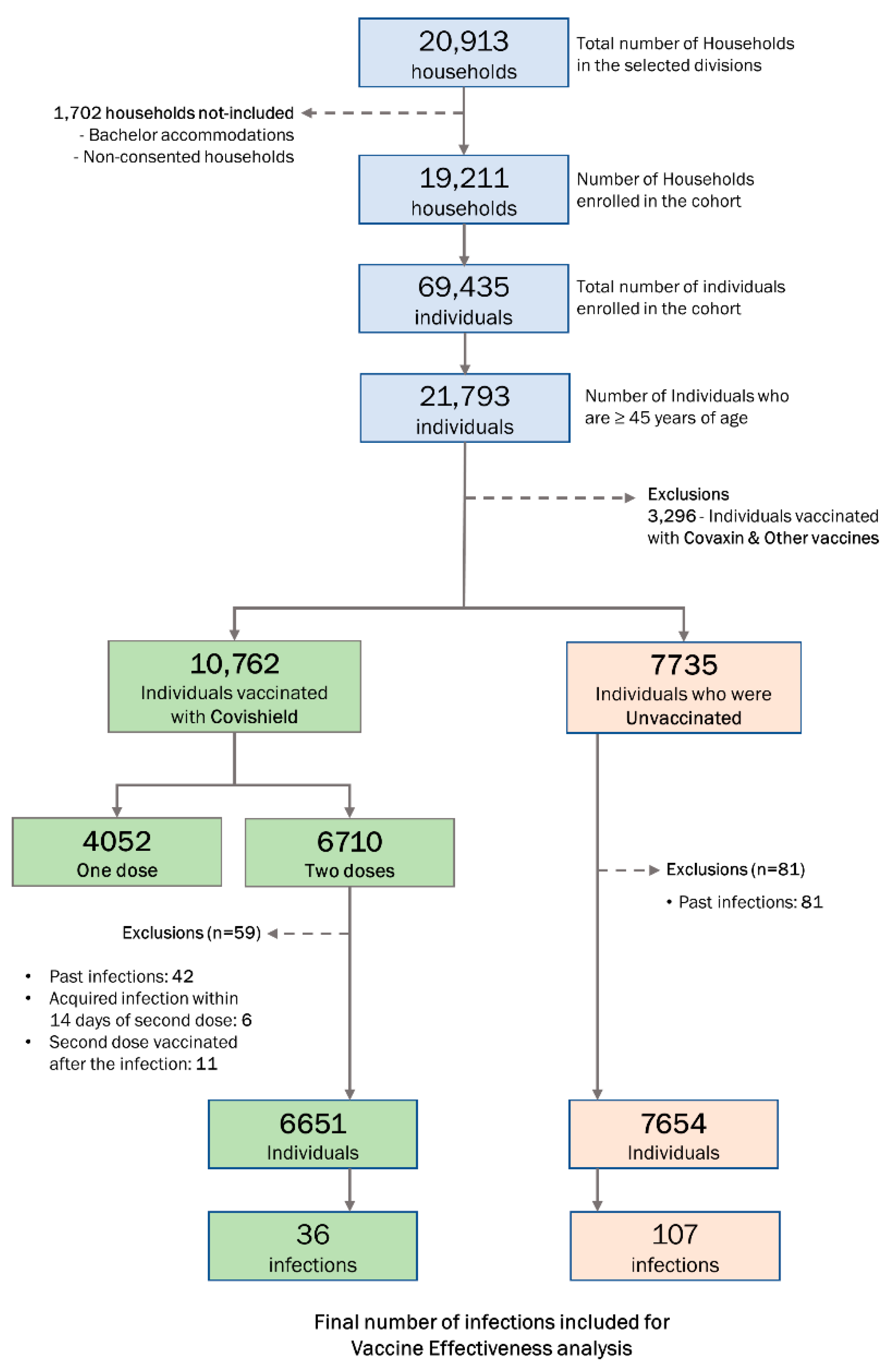
2.3. Sample Size and Sampling Strategy
2.4. Data Collection
2.4.1. Exposure Assessment
2.4.2. Outcome Measures
2.4.3. Sample Collection and Laboratory Investigations
2.5. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics
3.2. Vaccination Status
3.3. COVID-19 Disease Outcomes
3.4. VE of Two Doses of Covishield
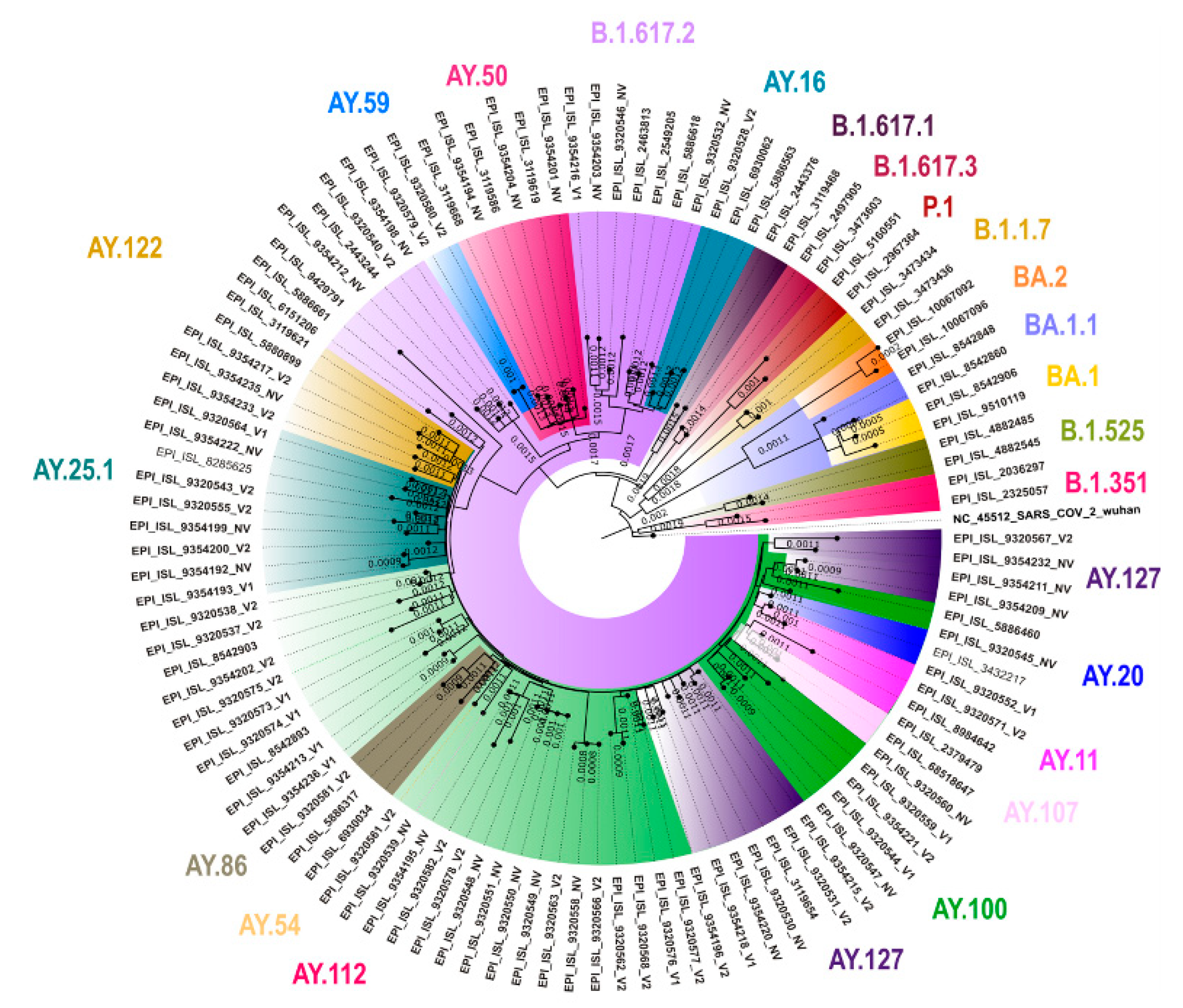
3.5. Genomic Sequencing Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 19 April 2021).
- Bhatia, R.; Abraham, P. Lessons Learnt During the first 100 days of COVID-19 Pandemic in India. Indian J. Med. Res. 2020, 151, 387–391. [Google Scholar] [CrossRef] [PubMed]
- MoHFW. Available online: https://www.mohfw.gov.in/ (accessed on 19 April 2021).
- COVID-19 Vaccine FAQs n.d. Available online: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html (accessed on 3 March 2022).
- Serum Institute of India-ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant)-COVISHIELD n.d. Available online: https://www.seruminstitute.com/product_covishield.php (accessed on 3 March 2022).
- COVAXIN-India’s First Indigenous Covid-19 Vaccine|Bharat Biotech n.d. Available online: https://www.bharatbiotech.com/covaxin.html (accessed on 3 March 2022).
- Delta Variant May Have Caused Widespread COVID-19 Infection in TN: Study. News Minute 2021. Available online: https://www.thenewsminute.com/article/delta-variant-may-have-caused-widespread-covid-19-infection-tn-study-150999 (accessed on 24 February 2022).
- Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials-The Lancet n.d. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00432-3/fulltext (accessed on 20 April 2021).
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Shankar, S.; Chatterjee, K.; Chatterjee, K.; Yadav, A.K.; Pandya, K.; Suryam, V.; Agrawal, S.; Ray, S.; Phutane, V.; et al. COVISHIELD (AZD1222) VaccINe effectiveness among healthcare and frontline Workers of INdian Armed Forces: Interim results of VIN-WIN cohort study. Med. J. Armed Forces India 2021, 77, S264–S270. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.J.; Mathews, K.P.; Paul, H.; Mammen, J.J.; Murugesan, M. Protective Effect of COVID-19 Vaccine Among Health Care Workers During the Second Wave of the Pandemic in India. Mayo Clin. Proc. 2021, 96, 2493–2494. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Awasthi, A.; Medigeshi, G.; Bhattacharya, S.; Mani, S.; Sivasubbu, S.; Shrivastava, T.; Samal, S.; Murugesan, D.R.; Desiraju, B.K.; et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: A test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect. Dis. 2021, 22, 473–482. [Google Scholar] [CrossRef]
- Jagadeesan, M.; Ganeshkumar, P.; Kaur, P.; Sriramulu, H.M.; Sakthivel, M.; Rubeshkumar, P.; Raju, M.; Murugesan, L.; Ganapathi, R.; Srinivasan, M.; et al. Epidemiology of COVID-19 and effect of public health interventions, Chennai, India, March–October 2020: An analysis of COVID-19 surveillance system. BMJ Open 2022, 12, e052067. [Google Scholar] [CrossRef]
- AZD1222 US Phase III Trial Met Primary Efficacy Endpoint in Preventing COVID-19 at Interim Analysis n.d. Available online: https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html (accessed on 20 April 2021).
- Safety and Immunogenicity of the ChAdOx1 nCoV-19 Vaccine Against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial-The Lancet n.d. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31604-4/fulltext (accessed on 20 April 2021).
- Sample Size Calculator for Evaluation of COVID-19 Vaccine Effectiveness (Excel) n.d. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccine_effectiveness-measurement_tool-2021.1 (accessed on 20 April 2021).
- Hartung, C.; Lerer, A.; Anokwa, Y.; Tseng, C.; Brunette, W.; Borriello, G. Open data kit: Tools to build information services for developing regions. ACM/IEEE Int. Conf. Inf. Commun. Technol. Dev. 2010, 18, 1–12. [Google Scholar] [CrossRef]
- Plumb, I.D.; Feldstein, L.R.; Barkley, E.; Posner, A.B.; Bregman, H.S.; Hagen, M.B.; Gerhart, J.L. Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19–Associated Hospitalization among Adults with Previous SARS-CoV-2 Infection—United States, June 2021–February 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef] [PubMed]
- United States of America; Department of Health & Human Resources, Nevada. Public Health Surveillance Death Definition: COVID-19 (Shrader, p. 1). Available online: https://dpbh.nv.gov/uploadedFiles/dpbhnvgov/content/Programs/OPHIE/Docs/COVID-Death-Definition.pdf (accessed on 23 February 2022).
- Potdar, V.; Choudhary, M.; Vipat, V.; Jadhav, S.; Basu, A.; Cherian, S.; Abraham, P. Development of in vitro transcribed RNA as positive control for laboratory diagnosis of SARS-CoV-2 in India. Indian J. Med. Res. 2020, 151, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Bhoyar, R.C.; Jain, A.; Sehgal, P.; Divakar, M.K.; Sharma, D.; Imran, M.; Jolly, B.; Ranjan, G.; Rophina, M.; Sharma, S.; et al. High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing. PLoS ONE 2021, 16, e0247115. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software; Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- England. Public Health. COVID-19 Vaccine Surveillance Report Week 38.:33. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1019992/Vaccine_surveillance_report_-_week_38.pdf (accessed on 3 March 2022).
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Chakeri, A.; Ioannidis, J.P.; Richter, L.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Allerberger, F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021, 51, e13520. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Klausner, J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2021, 22, 12–14. [Google Scholar] [CrossRef]
- Murhekar, M.; Bhatnagar, T.; Selvaraju, C.P. Monitoring the trend of SARS-CoV-2 seroprevalence in Chennai, India, July and October 2020. Trans. R. Soc. Trop. Med. Hyg. 2021, 11, 1350–1352. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Thangaraj, J.W.V.; Saravanakumar, V.; Kumar, M.S.; Selvaraju, S.; Rade, K.; Kumar, C.G.; Sabarinathan, R.; Turuk, A.; et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020–January 2021. Int. J. Infect. Dis. 2021, 108, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Serosurvey Finds Covid Antibodies in 66.2% of Tamil Nadu People; It’s 82% in Chennaiites. The Times of India. 021 Jul 31. Available online: https://timesofindia.indiatimes.com/city/chennai/serosurvey-finds-covid-antibodies-in-66-2-of-tamil-nadu-people-its-82-in-chennaiites/articleshow/84923223.cms (accessed on 6 June 2022).
- Jagadeesan, M.; Rubeshkumar, P.; Raju, M.; Sakthivel, M.; Murali, S.; Nagarajan, R.; Sendhilkumar, M.; Sambath, I.; Ilangovan, K.; Harikrishnan, D.; et al. Surveillance for face mask compliance, Chennai, Tamil Nadu, India, October-December 2020. PLoS ONE 2021, 16, e0257739. [Google Scholar] [CrossRef]
- Bhatnagar, T.; Chaudhuri, S.; Ponnaiah, M.; Yadav, P.D.; Sabarinathan, R.; Sahay, R.R.; Murhekar, M.V. Effectiveness of BBV152/Covaxin and AZD1222/Covishield Vaccines Against Severe COVID-19 and B.1.617.2/Delta Variant in India, 2021: A Multi-Centric Hospital-Based Case-Control Study; Social Science Research Network: Rochester, NY, USA, 2021. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Surveillance Reports (Weeks 19 to 38). GOVUK n.d. Available online: https://www.gov.uk/government/publications/covid-19-vaccine-surveillance-report (accessed on 26 May 2022).
| Characteristic | n | % | |
|---|---|---|---|
| Division | 147 | 8382 | 38.5 |
| 151 | 4881 | 22.4 | |
| 153 | 8530 | 39.1 | |
| Gender | Male | 10,916 | 50.1 |
| Female | 10,844 | 49.8 | |
| Transgender | 33 | 0.1 | |
| Age group (in years) | 45–59 | 13,114 | 60.2 |
| ≥60 | 8679 | 39.8 | |
| Education | No formal education | 2689 | 12.3 |
| School education | 11,749 | 54.0 | |
| College education | 7355 | 33.7 | |
| Occupation | Health care/Frontline worker | 532 | 2.4 |
| Others | 21,261 | 97.6 | |
| Comorbidity (N = 21,788) * | Known HT | 3770 | 17.3 |
| Known DM | 4251 | 19.5 | |
| Others | 904 | 4.1 | |
| Vaccination status | Unvaccinated | 7735 | 35.5 |
| Received only one dose | 4972 | 22.8 | |
| Received both the doses | 9086 | 41.7 | |
| Person per room | <3 | 14,755 | 67.7 |
| ≥3 | 7038 | 32.3 | |
| Residential Area | Slum | 3271 | 15.0 |
| Non-Slum | 18,522 | 85.0 | |
| HT—hypertension | |||
| DM—diabetes mellitus |
| Characteristic | N | Unvaccinated | Covishield | Covaxin | Other Vaccines | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One Dose | Two Doses | One Dose | Two Doses | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Overall | Overall | 21,793 | 7735 | 35.5 | 4052 | 18.6 | 6710 | 30.8 | 904 | 4.1 | 2357 | 10.8 | 35 | 0.2 |
| Gender | Male | 10,916 | 3725 | 34.1 | 2024 | 18.5 | 3498 | 32.0 | 419 | 3.8 | 1228 | 11.2 | 22 | 0.2 |
| Female /TG | 10,877 | 4010 | 36.9 | 2028 | 18.6 | 3212 | 29.5 | 485 | 4.5 | 1129 | 10.4 | 13 | 0.1 | |
| Age Group (in years) | 45–59 | 13,114 | 4759 | 36.3 | 2828 | 21.6 | 3656 | 27.9 | 570 | 4.3 | 1278 | 9.7 | 23 | 0.2 |
| ≥60 | 8679 | 2976 | 34.3 | 1224 | 14.1 | 3054 | 35.2 | 334 | 3.8 | 1079 | 12.4 | 12 | 0.1 | |
| Characteristic | Total | COVID-19 Positive | Incidence per 100,000 Population | Recovered without Oxygen | Severe Disease | Died | No Information | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | n | % | n | % | n | % | ||||
| Overall | 14,305 | 143 | 1000 | 115 | 80.4 | 6 | 4.2 | 5 | 3.5 | 17 | 11.9 | |
| Gender | Male | 7150 | 69 | 965 | 58 | 84.1 | 2 | 2.9 | 2 | 2.9 | 7 | 10.1 |
| Female/TG | 7155 | 74 | 1034 | 57 | 77.0 | 4 | 5.4 | 3 | 4.1 | 10 | 13.5 | |
| Age Group (in years) | 45–59 | 8341 | 86 | 1031 | 69 | 80.2 | 4 | 4.7 | 1 | 1.2 | 12 | 14.0 |
| ≥60 | 5964 | 57 | 956 | 46 | 80.7 | 2 | 3.5 | 4 | 7.0 | 5 | 8.8 | |
| Characteristics & Its Category | Vaccination Status | Total | COVID-19 Positive | RR (95% CI) | VE (95% CI) | Strata Adjusted RR * (95% CI) | Strata Adjusted VE * (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % | |||||||
| Overall | ≥45 years | Received two doses | 6651 | 36 | 0.5 | 0.386 (0.264–0.564) | 61.4 (43.6–73.6) | ||
| Unvaccinated | 7654 | 107 | 1.4 | ||||||
| Age Group (in years) | 45–59 | Received two doses | 3628 | 18 | 0.5 | 0.344 (0.205–0.577) | 65.6 (42.3–79.5) | ||
| Unvaccinated | 4713 | 68 | 1.4 | ||||||
| ≥60 | Received two doses | 3023 | 18 | 0.6 | 0.449 (0.258–0.783) | 55.1 (21.7–74.2) | |||
| Unvaccinated | 2941 | 39 | 1.3 | 0.386 (0.264–0.564) | 61.4 (43.6–73.6) | ||||
| Gender | Male | Received two doses | 3463 | 18 | 0.5 | 0.376 (0.220–0.642) | 62.4 (35.8–78.0) | ||
| Unvaccinated | 3687 | 51 | 1.4 | ||||||
| Female/TG | Received two doses | 3188 | 18 | 0.6 | 0.400 (0.236–0.679) | 60.0 (32.1–76.4) | |||
| Unvaccinated | 3967 | 56 | 1.4 | 0.388 (0.266–0.565) | 61.2 (43.5–73.4) | ||||
| Comorbidity | HT/DM | Received two doses | 1987 | 12 | 0.6 | 0.459 (0.231–0.916) | 54.1 (8.4–77.0) | ||
| Unvaccinated | 1826 | 24 | 1.3 | ||||||
| Non-HT/DM | Received two doses | 4663 | 24 | 0.5 | 0.361 (0.230–0.568) | 63.9 (43.2–77.0) | |||
| Unvaccinated | 5828 | 83 | 1.4 | 0.389 (0.266–0.567) | 61.1 (43.3–73.4) | ||||
| Person per room | <3 | Received two doses | 5034 | 31 | 0.6 | 0.389 (0.256–0.591) | 61.1 (40.9–74.4) | ||
| Unvaccinated | 4610 | 73 | 1.6 | ||||||
| ≥3 | Received two doses | 1617 | 5 | 0.3 | 0.277 (0.108–0.706) | 72.3 (29.4–89.2) | |||
| Unvaccinated | 3044 | 34 | 1.1 | 0.362 (0.247–0.532) | 63.8 (46.8–75.3) | ||||
| Residential Area | Slum | Received two doses | 528 | 0 | 0.0 | 0.091 (0.005–1.512) | 90.9 (-51.2–99.5) | ||
| Unvaccinated | 1683 | 18 | 1.1 | ||||||
| Non-Slum | Received two doses | 6123 | 36 | 0.6 | 0.394 (0.268–0.580) | 60.6 (42.0–73.2) | |||
| Unvaccinated | 5971 | 89 | 1.5 | 0.369 (0.251–0.541) | 63.1 (45.9–74.9) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, S.; Sakthivel, M.; Pattabi, K.; Venkatasamy, V.; Thangaraj, J.W.V.; Shete, A.; Varghese, A.J.; Arjun, J.; Kumar, C.P.G.; Yadav, P.D.; et al. Effectiveness of the ChAdOx1 nCoV-19 Coronavirus Vaccine (CovishieldTM) in Preventing SARS-CoV2 Infection, Chennai, Tamil Nadu, India, 2021. Vaccines 2022, 10, 970. https://doi.org/10.3390/vaccines10060970
Murali S, Sakthivel M, Pattabi K, Venkatasamy V, Thangaraj JWV, Shete A, Varghese AJ, Arjun J, Kumar CPG, Yadav PD, et al. Effectiveness of the ChAdOx1 nCoV-19 Coronavirus Vaccine (CovishieldTM) in Preventing SARS-CoV2 Infection, Chennai, Tamil Nadu, India, 2021. Vaccines. 2022; 10(6):970. https://doi.org/10.3390/vaccines10060970
Chicago/Turabian StyleMurali, Sharan, Manikandanesan Sakthivel, Kamaraj Pattabi, Vettrichelvan Venkatasamy, Jeromie Wesley Vivian Thangaraj, Anita Shete, Alby John Varghese, Jaganathan Arjun, Chethrapilly Purushothaman Girish Kumar, Pragya D Yadav, and et al. 2022. "Effectiveness of the ChAdOx1 nCoV-19 Coronavirus Vaccine (CovishieldTM) in Preventing SARS-CoV2 Infection, Chennai, Tamil Nadu, India, 2021" Vaccines 10, no. 6: 970. https://doi.org/10.3390/vaccines10060970
APA StyleMurali, S., Sakthivel, M., Pattabi, K., Venkatasamy, V., Thangaraj, J. W. V., Shete, A., Varghese, A. J., Arjun, J., Kumar, C. P. G., Yadav, P. D., Sahay, R., Majumdar, T., Dudhmal, M., Sivalingam, A., Dhanapal, S. R., Durai Samy, A., Radhakrishnan, V., Muni Krishnaiah, M. M., Arunachalam, S., ... Murhekar, M. (2022). Effectiveness of the ChAdOx1 nCoV-19 Coronavirus Vaccine (CovishieldTM) in Preventing SARS-CoV2 Infection, Chennai, Tamil Nadu, India, 2021. Vaccines, 10(6), 970. https://doi.org/10.3390/vaccines10060970






