Humoral and Cellular Immune Response in Asymptomatic Dogs with Visceral Leishmaniasis: A Review
Abstract
1. Introduction
2. Materials and Methods
- Studies performed in dogs.
- Studies that analyze humoral and/or cellular immune responses in serum and/or peripheral blood of asymptomatic dogs infected with L. donovani, L. chagasi, or L. infantum.
- Studies that include not only a group of asymptomatic dogs but also a group of healthy noninfected control dogs and/or symptomatic dogs with visceral leishmaniasis.
- Studies unrelated to visceral leishmaniasis.
- Studies that focused on human and nondog animal models.
- Studies in which dogs were vaccinated, immunized, or treated.
- Studies that did not focus on the immune response of dogs.
- Studies in which the immune response was analyzed in tissues.
- Studies in which the clinical classification was incorrect or inexistent.
3. Results
3.1. Phenotypic Characterization of the Lymphocyte Population
3.1.1. Cytolytic Activity of PBMCs
3.1.2. Nitric Oxide Production
3.1.3. Lymphoproliferative Response of PBMCs
3.1.4. Cytokine Profile
3.2. Analysis of the Humoral Immune Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; Da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Campino, L. Cytokine and Phenotypic Cell Profiles of Leishmania infantum Infection in the Dog. J. Trop. Med. 2012, 2012, 541571. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Moreno, J. Cytokine profiles in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Chen, Y.; Zhao, S.; Kuang, Y.; John Wu, C.H. Current Visceral Leishmaniasis Research: A Research Review to Inspire Future Study. Biomed Res. Int. 2018, 2018, 9872095. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.G.D.A.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Carneiro, C.M.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Francisco, A.F.; Cardoso, J.M.; Mathias, F.A.S.; et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 2014, 205, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Campino, L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet. Parasitol. 2008, 158, 274–287. [Google Scholar] [CrossRef]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W.; Tafuri, W.L.; Corrêa-Oliveira, R. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef]
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; da Mariano, R.M.S.; Silveira, P.; de Melo-Júnior, O.A.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Velez, R.; Gallego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Fernandes, C.B.; Junior, J.T.M.; de Jesus, C.; Souza, B.; Larangeira, D.F.; Fraga, D.B.M.; Veras, P.S.T.; Barrouin-Melo, S.M. Comparison of two commercial vaccines against visceral leishmaniasis in dogs from endemic areas: IgG, and subclasses, parasitism, and parasite transmission by xenodiagnosis. Vaccine 2014, 32, 1287–1295. [Google Scholar] [CrossRef]
- Bongiorno, G.; Paparcone, R.; Foglia Manzillo, V.; Oliva, G.; Cuisinier, A.; Gradoni, L. Veterinary Parasitology Vaccination with LiESP/QA-21 (CaniLeish ®) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs—A preliminary xenodiagnosis study. Vet. Parasitol. 2013, 197, 691–695. [Google Scholar] [CrossRef]
- Miró, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel Areas for Prevention and Control of Canine Leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar] [CrossRef]
- Mancianti, F.; Gramiccia, M.; Gradoni, L.; Pieri, S. Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 566–567. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. When is an “asymptomatic” dog asymptomatic? Vet. Parasitol. 2014, 202, 341–342. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Mirá, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 1–16. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Marques, M.J.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Moreira, N.D.; Vitoriano-Souza, J.; Vieira, P.M.; Carneiro, C.M.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; et al. Humoral and cellular immune responses in dogs with inapparent natural Leishmania infantum infection. Vet. J. 2011, 190, 43–47. [Google Scholar] [CrossRef][Green Version]
- Laurenti, M.D.; Rossi, C.N.; da Matta, V.L.R.; Tomokane, T.Y.; Corbett, C.E.P.; Secundino, N.F.C.; Pimenta, P.F.P.; Marcondes, M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet. Parasitol. 2013, 196, 296–300. [Google Scholar] [CrossRef]
- Guarga, J.L.; Lucientes, J.; Peribáñez, M.A.; Molina, R.; Gracia, M.J.; Castillo, J.A. Experimental infection of Phlebotomus perniciosus and determination of the natural infection rates of Leishmania infantum in dogs. Acta Trop. 2000, 77, 203–207. [Google Scholar] [CrossRef]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis—New concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef]
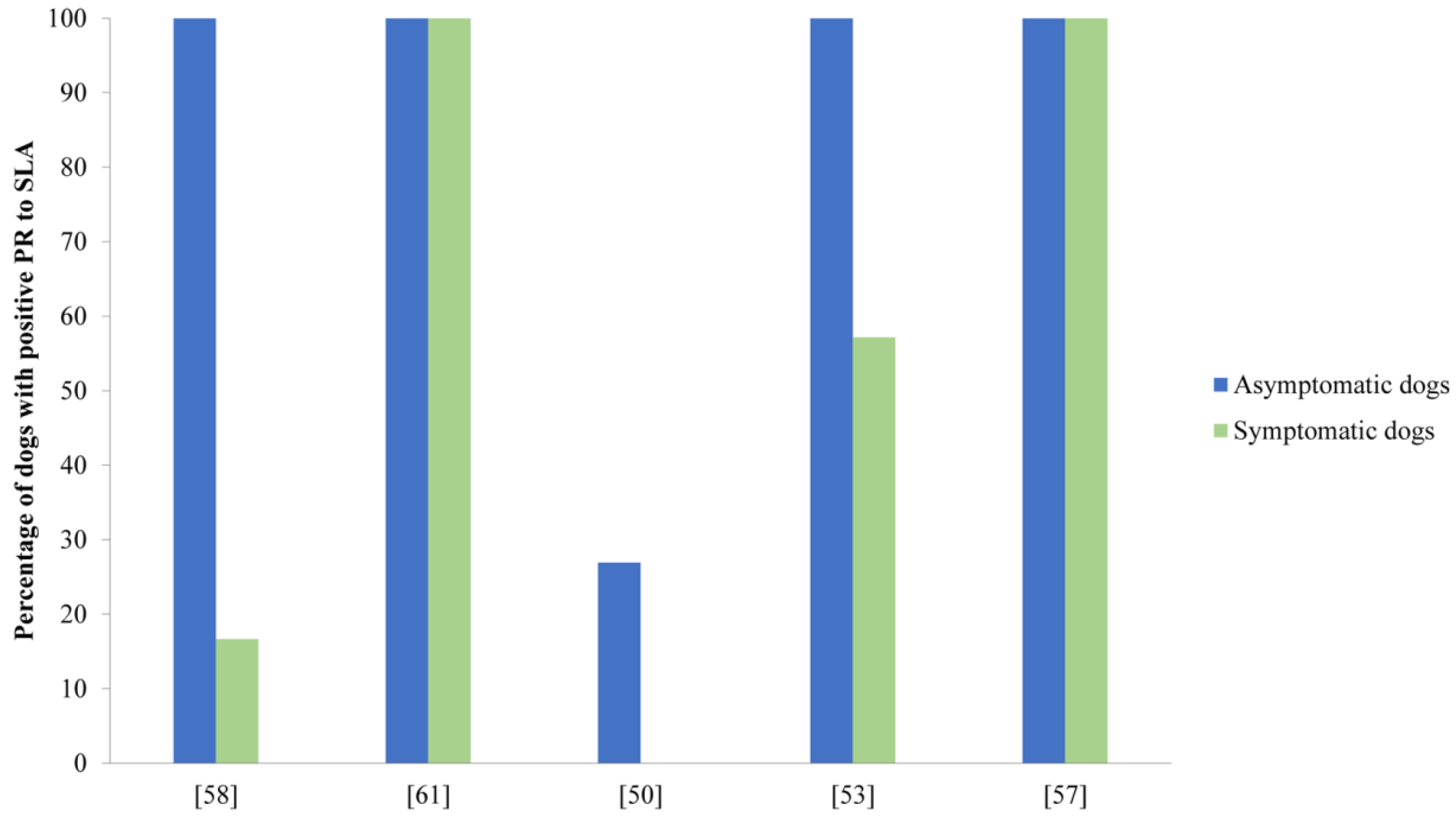
- Molina, R.; Amela, C.; Nieto, J.; San Andrés, M.; Gonzáles, F.; Castillo, J.A.; Lucientes, J.; Alvar, J. Infectivity of dogs infected with Leishmania infantum to colonized Phleobotomus perniciosus. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 491–493. [Google Scholar] [CrossRef]
- Guarga, J.L.; Moreno, J.; Lucientes, J.; Gracia, M.J.; Peribáñez, M.A.; Alvar, J.; Castillo, J.A. Canine leishmaniasis transmission: Higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Res. Vet. Sci. 2000, 249–253. [Google Scholar] [CrossRef]
- Vexenat, J.A.; De Castro, J.A.F.; Cavalcante, R.; Tavares, J.P.; Da Silva, M.R.B.; Batista, W.H.; Campos, J.H.F.; Howard, M.K.; Frame, I.; McNerney, R.; et al. Visceral leishmaniasis in Teresina, State of Piauí, Brazil: Preliminary observations on the detection and transmissibility of canine and sandfly infections. Mem. Inst. Oswaldo Cruz 1994, 89, 131–135. [Google Scholar] [CrossRef][Green Version]
- da Costa-Val, A.P.; Cavalcanti, R.R.; de Figueiredo Gontijo, N.; Michalick, M.S.M.; Alexander, B.; Williams, P.; Melo, M.N. Canine visceral leishmaniasis: Relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Vet. J. 2007, 174, 636–643. [Google Scholar] [CrossRef]
- Michalsky, É.M.; Rocha, M.F.; da Rocha Lima, A.C.V.M.; França-Silva, J.C.; Pires, M.Q.; Oliveira, F.S.; Pacheco, R.S.; dos Santos, S.L.; Barata, R.A.; Romanha, Á.J.; et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies o Carlos Franc. Vet. Parasitol. 2007, 147, 67–76. [Google Scholar] [CrossRef]
- Travi, B.L.; Tabares, C.J.; Cadena, H.; Ferro, C.; Osorio, Y. Canine visceral leishmaniasis in Colombia: Relationship between clinical and parasitological status and infectivity for sand flies. Am. Soc. Trop. Med. Hyg. 2001, 64, 119–124. [Google Scholar] [CrossRef]
- Alvar, J.; Molina, R.; San Andrés, M.; Tesouro, M.; Nieto, J.; Vitutia, M.; Gonzalés, F.; San Andrés, M.D.; Boggio, J.; Rodriguez, F.; et al. Canine leishmaniasis: Clinical, parasitological and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol. 1994, 88, 371–378. [Google Scholar] [CrossRef]
- Borja, L.S.; Sousa, O.M.F.; Solcà, M.D.S.; Bastos, L.A.; Bordoni, M.; Magalhães, J.T.; Larangeira, D.F.; Barrouin-Melo, S.M.; Fraga, D.B.M.; Veras, P.S.T. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet. Parasitol. 2016, 229, 110–117. [Google Scholar] [CrossRef]
- Alvar, J.; Can, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar] [CrossRef]
- Manna, L.; Reale, S.; Viola, E.; Vitale, F.; Manzillo, V.F.; Michele, P.L.; Caracappa, S.; Gravino, A.E. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Vet. Parasitol. 2006, 142, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Alves, F.; Bucheton, B.; Burrows, L.; Büscher, P.; Carrillo, E.; Felger, I.; Hübner, M.P.; Moreno, J.; Pinazo, M.J.; et al. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Semin. Immunopathol. 2020, 42, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Giunchetti, R.C.; Silveira, P.; Resende, L.A.; Leite, J.C.; Melo-Júnior, O.A.D.O.; Alves, M.L.R.; Costa, L.M.; Lair, D.F.; Chaves, V.R.; Soares, I.D.S.; et al. Canine visceral leishmaniasis biomarkers and their employment in vaccines. Vet. Parasitol. 2019, 271, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Barbiéri, C.L. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Reis, A.B.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Guerra, L.L.; Carvalho, M.G.; Mayrink, W.; Genaro, O.; Corrêa-Oliveira, R.; Martins-Filho, O.A. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clin. Exp. Immunol. 2006, 146, 303–311. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Alexandre-Pires, G.; Soares-Clemente, M.; Marques, C.; Rodrigues, O.R.; De Brito, T.V.; Da Fonseca, I.P.; Alves, L.C.; Santos-Gomes, G.M. Cytokine Gene Expression in the Tissues of Dogs Infected by Leishmania infantum. J. Comp. Pathol. 2011, 145, 336–344. [Google Scholar] [CrossRef]
- Nicolato, R.D.C.; De Abreu, R.T.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Reis, L.E.S.; Carvalho, M.D.G.; Carneiro, C.M.; Giunchetti, R.C.; Bouillet, L.E.M.; Lemos, D.S.; et al. Clinical forms of canine visceral leishmaniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. PLoS ONE 2013, 8, e82947. [Google Scholar] [CrossRef]
- Bourdoiseau, G.; Bonnefont, C.; Hoareau, E.; Boehringer, C.; Stolle, T.; Chabanne, L. Specific IgG1 and IgG2 antibody and lymphocyte subset levels in naturally Leishmania infantum-infected treated and untreated dogs. Vet. Immunol. Immunopathol. 1997, 59, 21–30. [Google Scholar] [CrossRef]
- Matralis, D.; Papadogiannakis, E.; Kontos, V.; Papadopoulos, E.; Ktenas, E.; Koutinas, A. Detection of intracellular IFN-γ and IL-4 cytokines in CD4+ and CD8+ T cells in the peripheral blood of dogs naturally infected with Leishmania infantum. Parasite Immunol. 2016, 38, 510–515. [Google Scholar] [CrossRef]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Rubino, V.; Piantedosi, D.; Di Loria, A.; Ruggiero, G.; Ciaramella, P.; Terrazzano, G. Regulatory T cells, Cytotoxic T lymphocytes and a Th1 cytokine profile in dogs naturally infected by Leishmania infantum. Res. Vet. Sci. 2013, 95, 942–949. [Google Scholar] [CrossRef]
- Rosypal, A.C.; Gogal, R.M.; Zajac, A.M.; Troy, G.C.; Lindsay, D.S. Flow cytometric analysis of cellular immune responses in dogs experimentally infected with a North American isolate of Leishmania infantum. Vet. Parasitol. 2005, 131, 45–51. [Google Scholar] [CrossRef]
- Alexandre-Pires, G.; de Brito, M.T.V.; Algueró, C.; Martins, C.; Rodrigues, O.R.; da Fonseca, I.P.; Santos-Gomes, G. Canine leishmaniosis. Immunophenotypic profile of leukocytes in different compartments of symptomatic, asymptomatic and treated dogs. Vet. Immunol. Immunopathol. 2010, 137, 275–283. [Google Scholar] [CrossRef]
- Schaut, R.G.; Lamb, I.M.; Toepp, A.J.; Scott, B.; Mendes-Aguiar, C.O.; Coutinho, J.F.V.; Jeronimo, S.M.B.; Wilson, M.E.; Harty, J.T.; Waldschmidt, T.J.; et al. Regulatory IgD hi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. J. Immunol. 2016, 196, 4100–4109. [Google Scholar] [CrossRef]
- Esch, K.J.; Juelsgaard, R.; Martinez, P.A.; Jones, D.E.; Petersen, C.A. Programmed Death 1–Mediated T Cell Exhaustion during Visceral Leishmaniasis Impairs Phagocyte Function. J. Immunol. 2013, 191, 5542–5550. [Google Scholar] [CrossRef]
- Pinelli, E.; Gonzalo, R.M.; Boog, C.J.P.; Rutten, V.P.M.G.; Gebhard, D.; Del Real, G.; Ruitenberg, E.J. Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. Eur. J. Immunol. 1995, 25, 1594–1600. [Google Scholar] [CrossRef]
- Panaro, M.A.; Brandonisio, O.; de Caprariis, D.; Cavallo, P.; Cianciulli, A.; Mitolo, V.; Otranto, D. Canine leishmaniasis in Southern Italy: A role for nitric oxide released from activated macrophages in asymptomatic infection? Parasites Vectors 2008, 1, 1–7. [Google Scholar] [CrossRef]
- da Costa Pinheiro, P.H.; de Souza Dias, S.; Dantas Eulálio, K.; Mendonça, I.L.; Katz, S.; Barbiéri, C.L. Recombinant cysteine proteinase from Leishmania (Leishmania) chagasi implicated in human and dog T-cell responses. Infect. Immun. 2005, 73, 3787–3789. [Google Scholar] [CrossRef]
- Souza, C.C.; Barreto, T.D.O.; da Silva, S.M.; Pinto, A.W.J.; Figueiredo, M.M.; Ferreira Rocha, O.G.; Cangussú, S.D.; Tafuri, W.L. A potential link among antioxidant enzymes, histopathology and trace elements in canine visceral leishmaniasis. Int. J. Exp. Pathol. 2014, 95, 260–270. [Google Scholar] [CrossRef]
- De Luna, R.; Vuotto, M.L.; Ielpo, M.T.L.; Ambrosio, R.; Piantedosi, D.; Moscatiello, V.; Ciaramella, P.; Scalone, A.; Gradoni, L.; Mancino, D. Early suppression of lymphoproliferative response in dogs with natural infection by Leishmania infantum. Vet. Immunol. Immunopathol. 1999, 70, 95–103. [Google Scholar] [CrossRef]
- Fernández-Pérez, F.J.; Gómez-Muñoz, M.T.; Méndez, S.; Alunda, J.M. Leishmania-specific lymphoproliferative responses and IgG1/IgG2 immunodetection patterns by Western blot in asymptomatic, symptomatic and treated dogs. Acta Trop. 2003, 86, 83–91. [Google Scholar] [CrossRef]
- Rafati, S.; Nakhaee, A.; Taheri, T.; Ghashghaii, A.; Salmanian, A.H.; Jimenez, M.; Mohebali, M.; Masina, S.; Fasel, N. Expression of cysteine proteinase type I and II of Leishmania infantum and their recognition by sera during canine and human visceral leishmaniasis. Exp. Parasitol. 2003, 103, 143–151. [Google Scholar] [CrossRef]
- Nakhaee, A.; Taheri, T.; Taghikhani, M.; Mohebali, M.; Salmanian, A.H.; Fasel, N.; Rafati, S. Humoral and cellular immune responses against Type I cysteine proteinase of Leishmania infantum are higher in asymptomatic than symptomatic dogs selected from a naturally infected population. Vet. Parasitol. 2004, 119, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Ahmed, S.; Goldsmith-Pestana, K.; Nieto, J.; Osorio, Y.; Travi, B.; Moreno, J.; McMahon-Pratt, D. Immunogenicity of the P-8 amastigote antigen in the experimental model of canine visceral leishmaniasis. Vaccine 2007, 25, 1534–1543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrillo, E.; Crusat, M.; Nieto, J.; Chicharro, C.; Thomas, M.D.C.; Martínez, E.; Valladares, B.; Cañavate, C.; Requena, J.M.; López, M.C.; et al. Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine 2008, 26, 1902–1911. [Google Scholar] [CrossRef][Green Version]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-Corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef]
- Rodríguez-Cortés, A.; Fernández-Bellón, H.; Ramis, A.; Ferrer, L.; Alberola, J.; Solano-Gallego, L. Leishmania-specific isotype levels and their relationship with specific cell-mediated immunity parameters in canine leishmaniasis. Vet. Immunol. Immunopathol. 2007, 116, 190–198. [Google Scholar] [CrossRef]
- Rodríguez-Cortés, A.; Ojeda, A.; López-Fuertes, L.; Timón, M.; Altet, L.; Solano-Gallego, L.; Sánchez-Robert, E.; Francino, O.; Alberola, J. A long term experimental study of canine visceral leishmaniasis. Int. J. Parasitol. 2007, 37, 683–693. [Google Scholar] [CrossRef]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; Del Real, G.; Ruitenberg, J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [CrossRef]
- Cabral, M.; O’Grady, J.E.; Gomes, S.; Sousa, J.C.; Thompson, H.; Alexander, J. The immunology of canine leishmaniosis: Strong evidence for a developing disease spectrum from asymptomatic dogs. Vet. Parasitol. 1998, 76, 173–180. [Google Scholar] [CrossRef]
- Rhalem, A.; Sahibi, H.; Guessous-Idrissi, N.; Lasri, S.; Natami, A.; Riyad, M.; Berrag, B. Immune response against Leishmania antigens in dogs naturally and experimentally infected with Leishmania infantum. Vet. Parasitol. 1999, 81, 173–184. [Google Scholar] [CrossRef]
- Leandro, C.; Santos-Gomes, G.M.; Campino, L.; Romão, P.; Cortes, S.; Rolão, N.; Gomes-Pereira, S.; Riça Capela, M.J.; Abranches, P. Cell mediated immunity and specific IgG1 and IgG2 antibody response in natural and experimental canine leishmaniosis. Vet. Immunol. Immunopathol. 2001, 79, 273–284. [Google Scholar] [CrossRef]
- Travi, B.L.; Osorio, E.Y.; Saldarriaga, O.A.; Cadena, H.; Tabares, C.J.; Peniche, A.; Lee, S.; Melby, P.C. Clinical, parasitologic, and immunologic evolution in dogs experimentally infected with sand fly-derived Leishmania chagasi promastigotes. Am. J. Trop. Med. Hyg. 2009, 81, 994–1003. [Google Scholar] [CrossRef]
- Chamizo, C.; Moreno, J.; Alvar, J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet. Immunol. Immunopathol. 2005, 103, 67–75. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Montserrrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-specific production of IFN-γ and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasites Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Abbehusen, M.M.C.; Dos Anjos Almeida, V.; Da, S.M.; Da Silva Pereira, L.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci. Rep. 2017, 7, 15914. [Google Scholar] [CrossRef]
- Solcà, M.S.; Andrade, B.B.; Abbehusen, M.M.C.; Teixeira, C.R.; Khouri, R.; Valenzuela, J.G.; Kamhawi, S.; Bozza, P.T.; Fraga, D.B.M.; Borges, V.M.; et al. Circulating Biomarkers of Immune Activation, Oxidative Stress and Inflammation Characterize Severe Canine Visceral Leishmaniasis. Sci. Rep. 2016, 6, 32619. [Google Scholar] [CrossRef]
- Panaro, M.A.; Brandonisio, O.; Cianciulli, A.; Cavallo, P.; Lacasella, V.; Paradies, P.; Testini, G.; De Caprariis, D.; Mitolo, V.; Otranto, D. Cytokine expression in dogs with natural Leishmania infantum infection. Parasitology 2009, 136, 823–831. [Google Scholar] [CrossRef]
- Vida, B.; Toepp, A.; Schaut, R.G.; Esch, K.J.; Juelsgaard, R.; Shimak, R.M.; Petersen, C.A. Immunologic progression of canine leishmaniosis following vertical transmission in United States dogs. Vet. Immunol. Immunopathol. 2016, 169, 34–38. [Google Scholar] [CrossRef]
- da Solcà, M.S.; Arruda, M.R.; Leite, B.M.M.; Mota, T.F.; Rebouças, M.F.; de Jesus, M.S.; Amorim, L.D.A.F.; Borges, V.M.; Valenzuela, J.; Kamhawi, S.; et al. Immune response dynamics and lutzomyia longipalpis exposure characterize a biosignature of visceral leishmaniasis susceptibility in a canine cohort. PLoS Negl. Trop. Dis. 2021, 15, 1–22. [Google Scholar] [CrossRef]
- Reis, A.B.; Teixeira-Carvalho, A.; Vale, A.M.; Marques, M.J.; Giunchetti, R.C.; Mayrink, W.; Guerra, L.L.; Andrade, R.A.; Corrêa-Oliveira, R.; Martins-Filho, O.A. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2006, 112, 102–116. [Google Scholar] [CrossRef]
- Oliveira, T.M.F.S.; Mineo, T.W.P.; Bason, M.; Day, M.J.; Machado, R.Z. IgG subclass profile of serum antibodies to Leishmania chagasi in naturally infected and vaccinated dogs. Vet. Parasitol. 2009, 162, 16–22. [Google Scholar] [CrossRef]
- Neto, R.G.T.; Giunchetti, R.C.; Carneiro, C.M.; de Vitor, R.W.A.; Coura-Vital, W.; Quaresma, P.F.; Ker, H.G.; de Melo, L.A.; Gontijo, C.M.F.; Reis, A.B. Relationship of Leishmania-specific IgG levels and IgG avidity with parasite density and clinical signs in canine leishmaniasis. Vet. Parasitol. 2010, 169, 248–257. [Google Scholar] [CrossRef]
- De Freitas, J.C.C.; Lopes-Neto, B.E.; De Abreu, C.R.A.; Coura-Vital, W.; Braga, S.L.; Reis, A.B.; Nunes-Pinheiro, D.C.S. Profile of anti-Leishmania antibodies related to clinical picture in canine visceral leishmaniasis. Res. Vet. Sci. 2012, 93, 705–709. [Google Scholar] [CrossRef]
- Iniesta, L.; Gállego, M.; Portús, M. Immunoglobulin G and E responses in various stages of canine leishmaniosis. Vet. Immunol. Immunopathol. 2005, 103, 77–81. [Google Scholar] [CrossRef]
- Laranjeira, D.F.; Da Matta, V.L.R.; Tomokane, T.Y.; Marcondes, M.; Corbet, C.E.P.; Laurenti, M.D. Serological and infection statuses of dogs from a visceral leishmaniasis-endemic area. Rev. Saude Publica 2014, 48, 563–570. [Google Scholar] [CrossRef]
- Lima, L.V.D.R.; Carneiro, L.A.; Campos, M.B.; Dos Santos, T.V.; Ramos, P.K.; Laurenti, M.D.; Teixeira, C.E.C.; Silveira, F.T. Further evidence associating IgG1, but not IgG2, with susceptibility to canine visceral leishmaniasis caused by Leishmania (L.) infantum chagasi-infection. Parasite 2017, 24, 37. [Google Scholar] [CrossRef]
- Nieto, C.G.; García-Alonso, M.; Requena, J.M.; Mirón, C.; Soto, M.; Alonso, C.; Navarrete, I. Analysis of the humoral immune response against total and recombinant antigens of Leishmania infantum: Correlation with disease progression in canine experimental leishmaniasis. Vet. Immunol. Immunopathol. 1999, 67, 117–130. [Google Scholar] [CrossRef]
- Campos, M.P.; Figueiredo, F.B.; Morgado, F.N.; dos Renzetti, A.R.S.; de Souza, S.M.M.; Pereira, S.A.; Rodrigues-Da-Silva, R.N.; Lima-Junior, J.D.C.; de Luca, P.M. Leishmania infantum virulence factor A2 protein: Linear B-cell epitope mapping and identification of three main linear B-cell epitopes in vaccinated and naturally infected dogs. Front. Immunol. 2018, 9, 1690. [Google Scholar] [CrossRef]
- Agallou, M.; Athanasiou, E.; Samiotaki, M.; Panayotou, G.; Karagouni, E. Identification of immunoreactive Leishmania infantum protein antigens to asymptomatic dog sera through combined immunoproteomics and bioinformatics analysis. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Riera, C.; Roura, X.; Iniesta, L.; Gallego, M.; Valladares, J.E.; Fisa, R.; Castillejo, S.; Alberola, J.; Ferrer, L.; et al. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas: Evolution in the course of infection and after treatment. Vet. Parasitol. 2001, 96, 265–276. [Google Scholar] [CrossRef]
- Cordeiro-da-Silva, A.; Cardoso, L.; Araújo, N.; Castro, H.; Tomás, A.; Rodrigues, M.; Cabral, M.; Vergnes, B.; Sereno, D.; Ouaissi, A. Identification of antibodies to Leishmania silent information regulatory 2 (SIR2) protein homologue during canine natural infections: Pathological implications. Immunol. Lett. 2003, 86, 155–162. [Google Scholar] [CrossRef]
- Almeida, M.A.O.; Jesus, E.E.V.; Sousa-Atta, M.L.B.; Alves, L.C.; Berne, M.E.A.; Atta, A.M. Antileishmanial antibody profile in dogs naturally infected with Leishmania chagasi. Vet. Immunol. Immunopathol. 2005, 106, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Schallig, H.D.F.H.; Cordeiro-da-Silva, A.; Cabral, M.; Alunda, J.M.; Rodrigues, M. Anti-Leishmania humoral and cellular immune responses in naturally infected symptomatic and asymptomatic dogs. Vet. Immunol. Immunopathol. 2007, 117, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, L.; Gállego, M.; Portús, M. Idiotype expression of IgG1 and IgG2 in dogs naturally infected with Leishmania infantum. Vet. Immunol. Immunopathol. 2007, 119, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Courtenay, O.; Garcez, L.M.; Kaye, P.M.; Shaw, M.A.; Dye, C.; Day, M.J. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2003, 91, 161–168. [Google Scholar] [CrossRef]
- Carson, C.; Quinnell, R.J.; Day, M.J.; Courtenay, O. Comparison of monoclonal and polyclonal antibodies for the detection of canine IgG1 and IgG2, and associations with infection outcome in Leishmania infantum naturally infected dogs. Vet. Immunol. Immunopathol. 2010, 133, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.C.; de Schubach, A.O.; Mouta-Confort, E.; Pacheco, T.M.V.; de Madeira, M.F.; de Abboud, L.C.S.; de Honse, C.O.; Alves, A.S.; Marzochi, M.C.A. Use of ELISA employing homologous and heterologous antigens for the dectection of IgG and subclasses (IgG1 and IgG2) in the diagnosis of Canine Visceral Leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 283–289. [Google Scholar] [CrossRef][Green Version]
- Chaabouni, A.; Elandoulsi, R.B.; Mhadhbi, M.; Gharbi, M.; Sassi, A. Comparative analysis of the Leishmania infantum-specific antibody repertoires and the autoantibody repertoires between asymptomatic and symptomatic dogs. Vet. Parasitol. 2018, 261, 9–17. [Google Scholar] [CrossRef]
- Maia, A.C.R.G.; Porcino, G.N.; Faria-Pinto, P.; Mendes, T.V.; Antinarelli, L.M.R.; Coimbra, E.S.; Reis, A.B.; Juliano, L.; Juliano, M.A.; Marques, M.J.; et al. Leishmania infantum nucleoside triphosphate diphosphohydrolase 1 (NTPDase 1) B-domain: Antibody antiproliferative effect on the promastigotes and IgG subclass responses in canine visceral leishmaniasis. Vet. Parasitol. 2019, 271, 38–44. [Google Scholar] [CrossRef]
- Marcondes, M.; Ikeda, F.A.; Vieira, R.F.C.; Day, M.J.; Lima, V.M.F.; Rossi, C.N.; Perri, S.H.V.; Biondo, A.W. Temporal IgG subclasses response in dogs following vaccination against Leishmania with Leishmune ®. Vet. Parasitol. 2011, 181, 153–159. [Google Scholar] [CrossRef]
- De Morais, C.G.V.; Castro Lima, A.K.; Terra, R.; Dos Santos, R.F.; Da-Silva, S.A.G.; Dutra, P.M.L. The dialogue of the host-parasite relationship: Leishmania spp. and Trypanosoma cruzi Infection. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Gomes-Pereira, S.; Rodrigues, O.R.; Santos-Gomes, G.M. Dynamics of CD62L/CD45RB CD4+ and CD8+ lymphocyte subsets in hepatic and splenic tissues during murine visceral leishmaniasis. Immunol. Lett. 2004, 95, 63–70. [Google Scholar] [CrossRef]
- Bunn, P.T.; de Oca, M.M.; de Labastida Rivera, F.; Kumar, R.; Ng, S.S.; Edwards, C.L.; Faleiro, R.J.; Sheel, M.; Amante, F.H.; Frame, T.C.M.; et al. Distinct Roles for CD4 + Foxp3 + Regulatory T Cells and IL-10–Mediated Immunoregulatory Mechanisms during Experimental Visceral Leishmaniasis Caused by Leishmania donovani. J. Immunol. 2018, 201, 3362–3372. [Google Scholar] [CrossRef]
- Ansari, N.A.; Ramesh, V.; Salotra, P. Interferon (IFN)-γ, tumor necrosis factor-α, interleukin-6, and IFN-γ receptor 1 are the major immunological determinants associated with post-kala azar dermal leishmaniasis. J. Infect. Dis. 2006, 194, 958–965. [Google Scholar] [CrossRef]
- Colpitts, S.L.; Dalton, N.M.; Scott, P. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J. Immunol. 2009, 182, 5702–5711. [Google Scholar] [CrossRef]
- Costa, S.F.; Gomes, V.O.; Maciel, M.O.D.S.; Melo, L.M.; Venturin, G.L.; Bragato, J.P.; Rebech, G.T.; de Santos, C.O.; de Oliveira, B.M.N.; de Sá Oliveira, G.G.; et al. Combined in vitro il-12 and il-15 stimulation promotes cellular immune response in dogs with visceral leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, 1–21. [Google Scholar] [CrossRef]
- Milano, S.; Di Bella, G.; D’Agostino, P.; Barbera, C.; Caruso, R.; La Rosa, M.; Ferlazzo, V.; Vitale, G.; La Russa, C.; Gambino, G.; et al. IL-15 in human visceral leishmaniasis caused by Leishmania infantum. Clin. Exp. Immunol. 2002, 127, 360–365. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Baneth, G. Canine leishmaniosis—A challenging zoonosis. Eur. J. Companion Anim. Pract. 2008, 18, 232–241. [Google Scholar]
- Cabral, M.; O’Grady, J.; Alexander, J. Demonstration of Leishmania specific cell mediated and humoral immunity in asymptomatic dogs. Parasite Immunol. 1992, 14, 531–539. [Google Scholar] [CrossRef]

| Cell Type Analyzed | Common Markers Studied | Additional Markers Studied | Clinical Classification (Number of Dogs | Geographical Location | Type of Infection | Methods | Main Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| T and B lymphocytes | CD3/CD5, CD4, CD8 | Foxp3 | AD (n = 23 SD (n = 22) CD (n = 30) | Campania region (Italy) | Natural | IFA and FC with mAb | No differences between AD and SD for any marker. ↓% CD4+CD3+ cells and ↑ % CD8+CD3+ cells in AD and SD versus CD (****). ↓ Treg CD4+ cells in AD and SD versus CD (****). | [40] |
| MHC-II | AD (n = 10) SD (n = 10) TD (n = 10) CD (n = 10) | Unknown | Natural | FC | ↓ CD3+ T cells in SD versus AD and CD (*). Similar numbers of CD4+ and CD8+ T cells between groups. ↑ % MHC-II+ lymphocytes in SD versus CD (*). | [42] | ||
| CD21, MHC-II, CD45RA, CD45RB | AD (n = 12) OD (n = 12) SD (n = 16) CD (n = 20) | Belo Horizonte (Brazil) | Natural | FC with mAb | ↑ CD5+, CD4+ and CD8+ cells in AD versus SD (**). ↓ CD21+ cells in SD versus AD (**) and CD (*). ↑ MHC-II expression and CD45RB/CD45RA ratio in lymphocytes from AD versus SD, OD, and CD (*). | [35] | ||
| CD21 | AD (n = 6) SD/TSD (n = 8) CD (n = 22) | France | Natural | FC with mAb | ↓ CD5+, CD4+, CD8+ and CD21+ cells in SD versus AD. ↓ CD21+ in AD versus CD (****). | [38] | ||
| AD-I (n = 8) AD-II (n = 10) SD (n = 16) CD (n = 7) | Belo Horizonte (Brazil) | Natural | FC with mAb | ↑ CD5+ and CD4+ cells in AD-I and AD-II versus SD (*). ↑ CD8+ cells in AD-II versus SD and CD (*). ↑ CD21+ cells in AD-II and CD versus SD (*). | [18] | |||
| AD-I (n = 34) AD-II (n = 20) OD (n = 8) SD (n = 42) CD (n = 28) | Belo Horizonte (Brazil) | Natural | FC with mAb | ↓ CD5+, CD4+, CD8+ and CD21+ cells in AD-II and SD versus AD-I and CD (****). | [6] | |||
| T lymphocytes | CD4, CD8 | - | AD (n = 4) SD (n = 8) CD (n = 2) | Virginia (USA) | Experimental | FC with mAb | Similar numbers of CD4+ and CD8+ T cells between groups. | [41] |
| AD (n = 20) SD (n = 20) CD (n = 20) | Atenas (Greece) | Natural | FC with mAb | ↑ CD4+ T cells in AD and CD versus SD (***). | [39] | |||
| B lymphocytes | CD19, CD21, IgD, IgM | - | AD (n = 7) SD (n = 7) CD (n = 7) | USA and Natal (Brazil) | Natural | FC and FACS | Similar % CD19+ and CD21+ cells between groups. ↑ IgDhi B cells in SD versus AD and CD (***). | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Castro, A.; Egui, A.; Thomas, M.C.; López, M.C. Humoral and Cellular Immune Response in Asymptomatic Dogs with Visceral Leishmaniasis: A Review. Vaccines 2022, 10, 947. https://doi.org/10.3390/vaccines10060947
García-Castro A, Egui A, Thomas MC, López MC. Humoral and Cellular Immune Response in Asymptomatic Dogs with Visceral Leishmaniasis: A Review. Vaccines. 2022; 10(6):947. https://doi.org/10.3390/vaccines10060947
Chicago/Turabian StyleGarcía-Castro, Ana, Adriana Egui, María Carmen Thomas, and Manuel Carlos López. 2022. "Humoral and Cellular Immune Response in Asymptomatic Dogs with Visceral Leishmaniasis: A Review" Vaccines 10, no. 6: 947. https://doi.org/10.3390/vaccines10060947
APA StyleGarcía-Castro, A., Egui, A., Thomas, M. C., & López, M. C. (2022). Humoral and Cellular Immune Response in Asymptomatic Dogs with Visceral Leishmaniasis: A Review. Vaccines, 10(6), 947. https://doi.org/10.3390/vaccines10060947





