Safety of Vaccines against SARS-CoV-2 among Polish Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
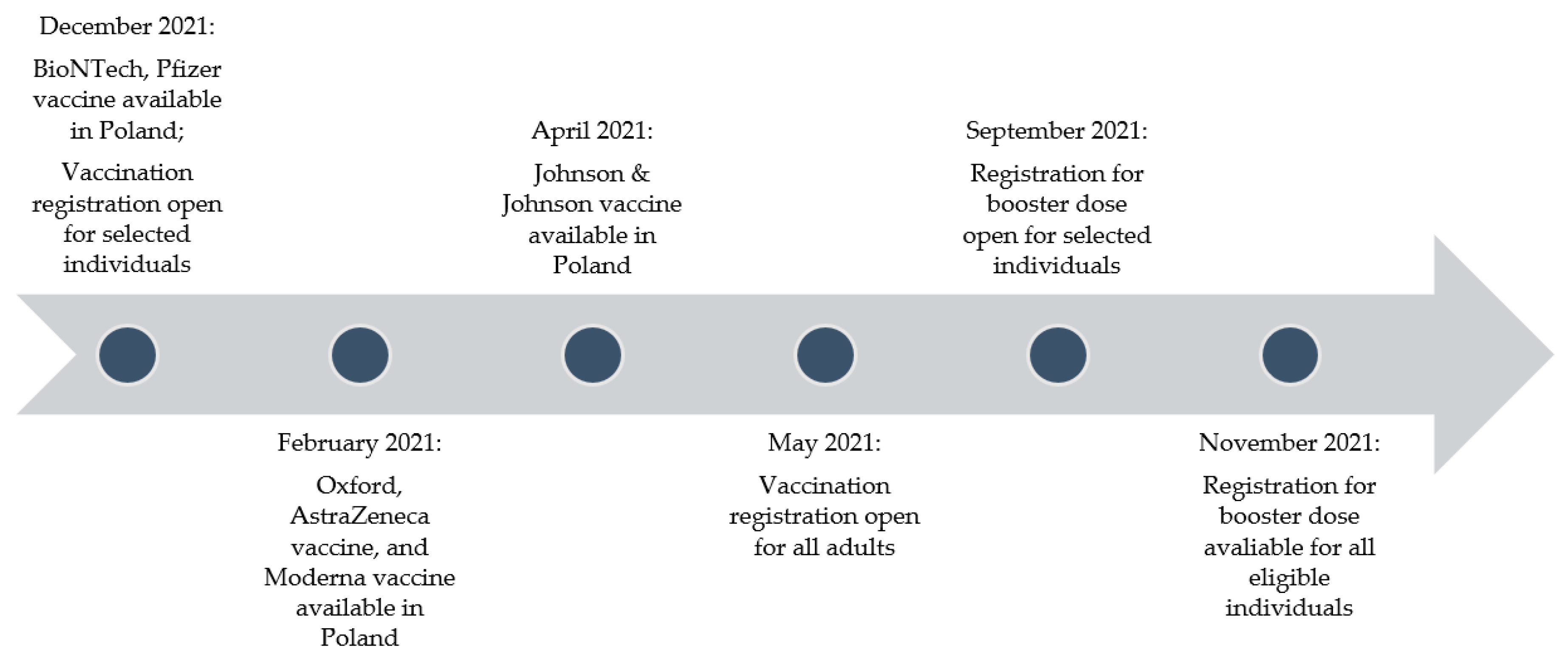
2.2. Timeline of Vaccination in Poland
2.3. Analyses
2.4. Standard Protocol Approvals
3. Results
3.1. Descriptive Statistics of the Study Population
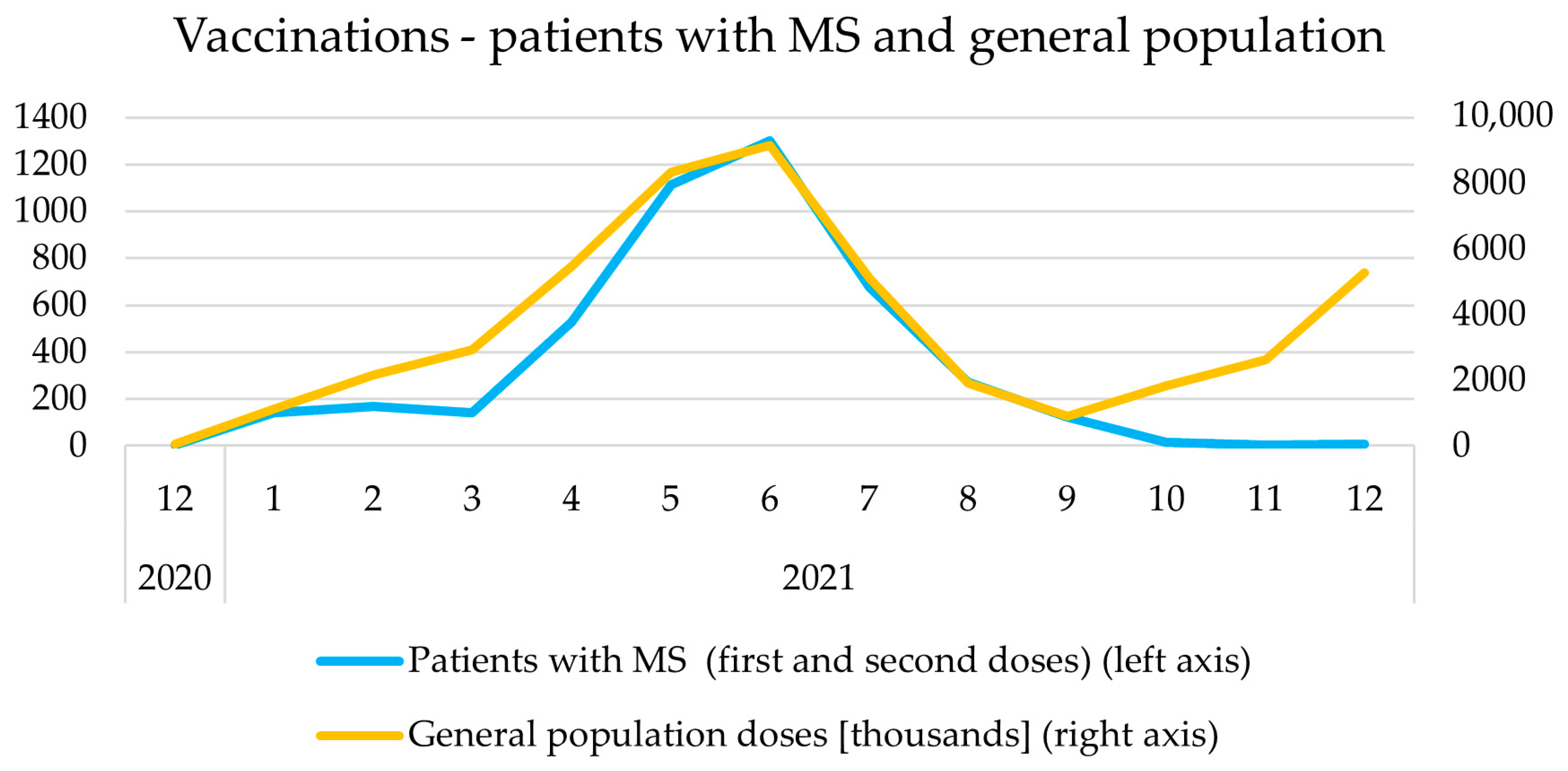
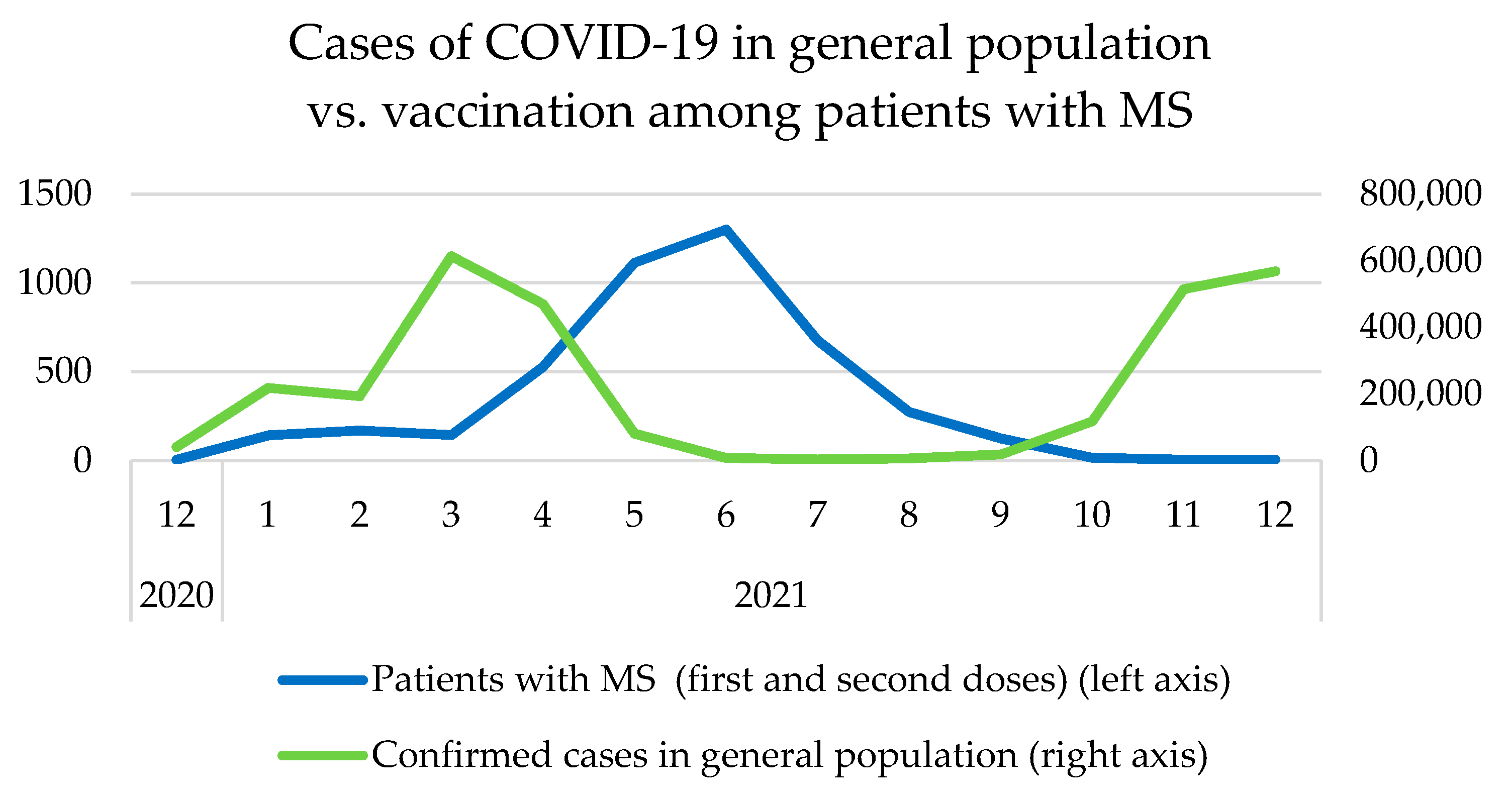
3.2. Timeline
3.3. Adverse Events
3.4. Disease-Modifying Therapies
3.5. Adverse Events and Vaccine Type
3.6. Adverse Events and Age
3.7. Adverse Events and Comorbid Diseases
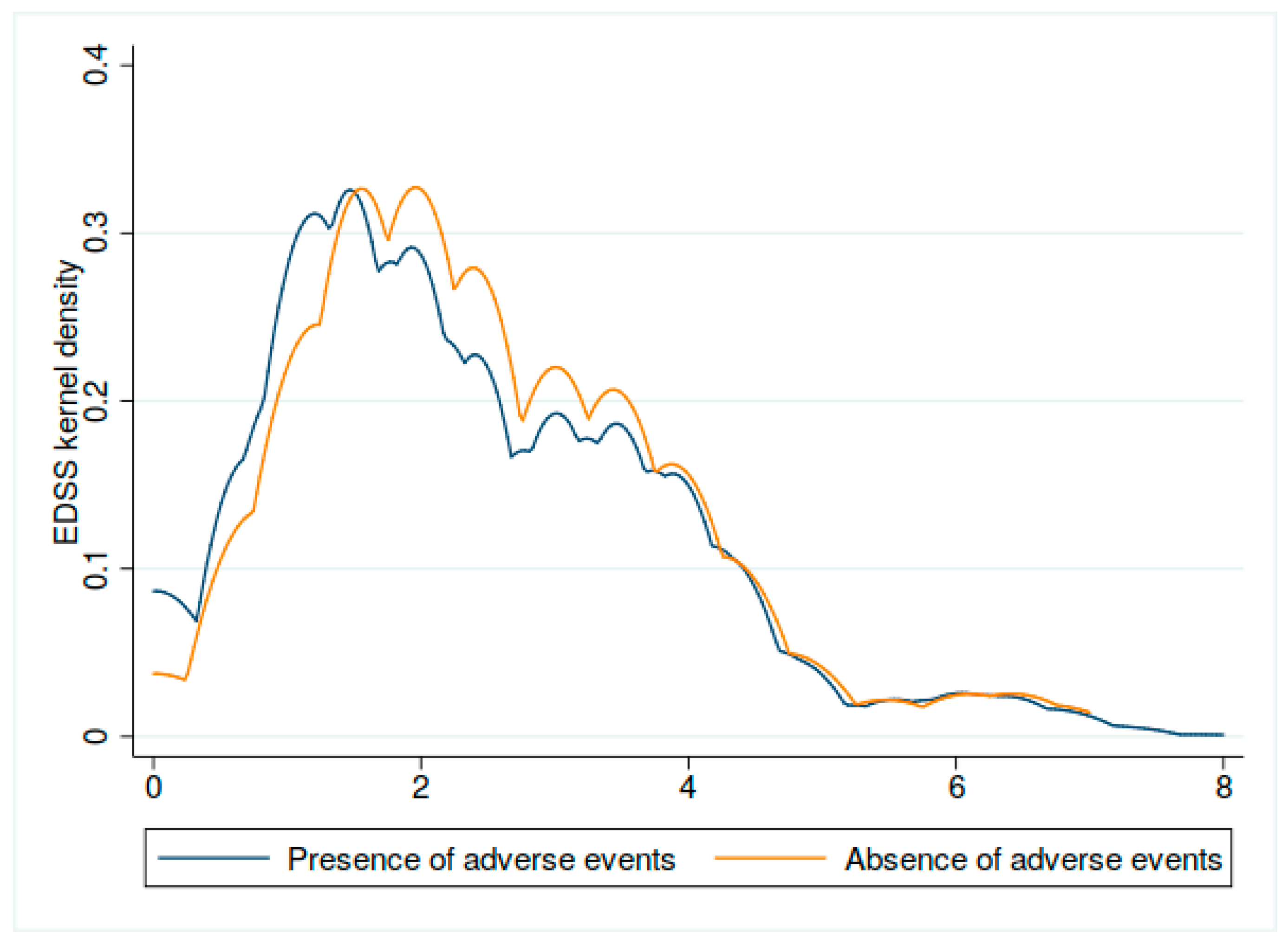
3.8. Adverse Events and EDSS
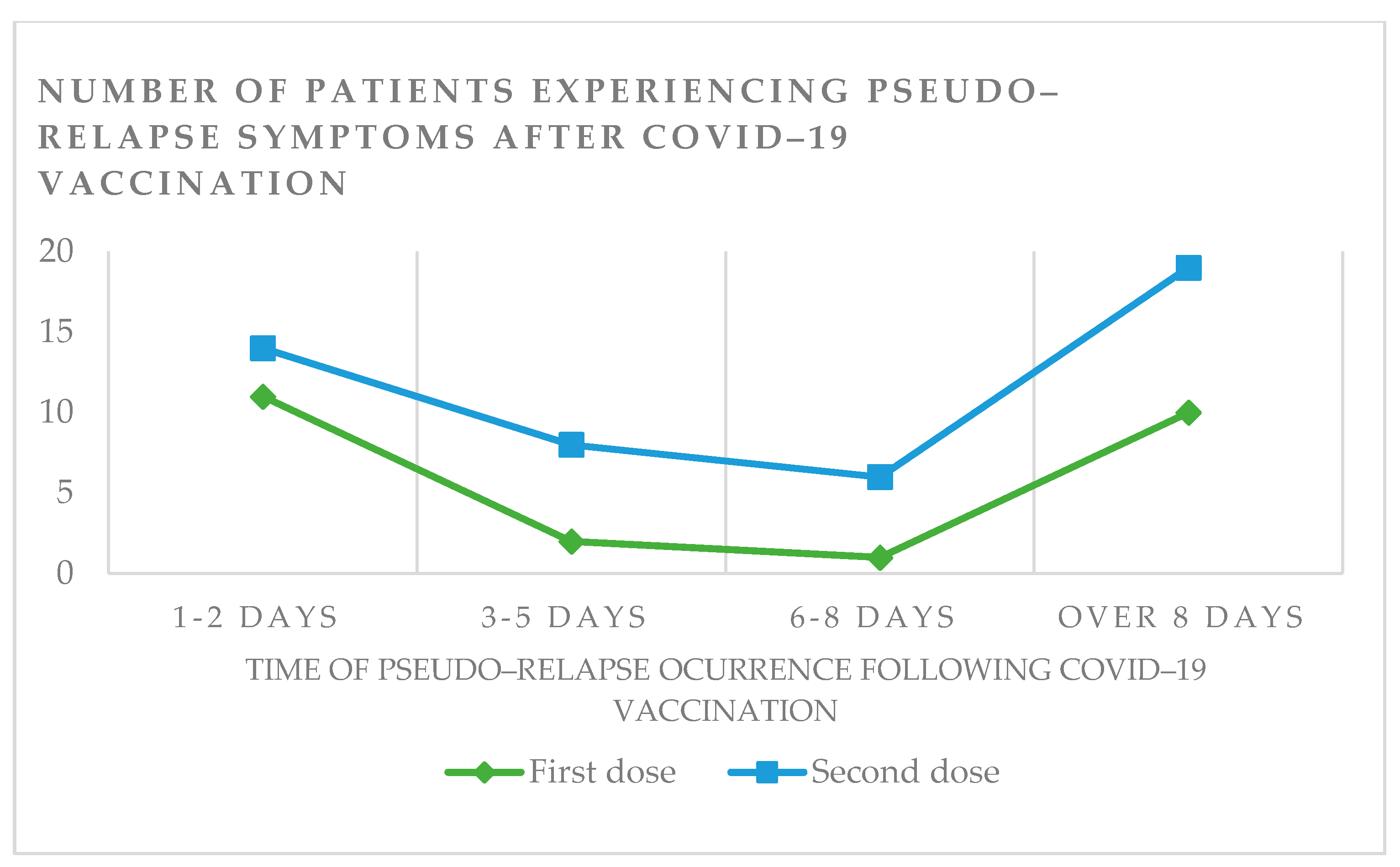
3.9. Adverse Events and Neurological Worsening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Pandemic. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 February 2022).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. Available online: https://pubmed.ncbi.nlm.nih.gov/34002089 (accessed on 2 February 2022). [CrossRef] [PubMed]
- COVID-19 Vaccine Guidance for People Living with MS. National Multiple Sclerosis Society. Available online: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance (accessed on 25 February 2022).
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.-N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. J. 2021, 27, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Nojszewska, M.; Kalinowska, A.; Adamczyk-Sowa, M.; Kułakowska, A.; Bartosik-Psujek, H. COVID-19 mRNA Vaccines (Pfizer-BioNTech and Moderna) in Patients with Multiple Sclerosis: A Statement by a Working Group Convened by the Section of Multiple Sclerosis and Neuroimmunology of the Polish Neurological Society. Neurol. Neurochir. Pol. 2021, 55, 8–11. Available online: https://pubmed.ncbi.nlm.nih.gov/33555604 (accessed on 3 February 2022). [CrossRef] [PubMed]
- MS Treatment Guidelines during the Coronavirus Pandemic. National MS Society. National Multiple Sclerosis Society. Available online: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/ms-treatment-guidelines-during-coronavirus (accessed on 3 February 2022).
- Capone, F.; Lucchini, M.; Ferraro, E.; Bianco, A.; Rossi, M.; Cicia, A.; Cortese, A.; Cruciani, A.; De Arcangelis, V.; De Giglio, L.; et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garjani, A.; Patel, S.; Bharkhada, D.; Rashid, W.; Coles, A.; Law, G.R.; Evangelou, N. Impact of Mass Vaccination on SARS-CoV-2 Infections among Multiple Sclerosis Patients Taking Immunomodulatory Disease-Modifying Therapies in England. Mult. Scler. Relat. Disord. 2022, 57, 103458. Available online: https://pubmed.ncbi.nlm.nih.gov/34896876 (accessed on 16 February 2022). [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum. Vaccines Immunother. 2021, 17, 3481–3483. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raport Zakażeń Koronawirusem (SARS-CoV-2)—Koronawirus: Informacje I Zalecenia—Portal Gov.pl. Available online: https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2 (accessed on 11 October 2021).
- Aktualności—Szczepienie Przeciwko COVID-19—Portal Gov.pl. Available online: https://www.gov.pl/web/szczepimysie/aktualnosci (accessed on 29 April 2022).
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017; Available online: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs (accessed on 5 February 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. Available online: https://pubmed.ncbi.nlm.nih.gov/34439984 (accessed on 15 February 2022). [CrossRef] [PubMed]
- Wagner, A.; Weinberger, B. Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives. Front. Immunol. 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Valerio, J.A.; Skinner-Taylor, C.M.; Moreno-Arquieta, I.A.; la Garza, J.A.C.-D.; Garcia-Arellano, G.; Gonzalez-Garcia, P.L.; Almaraz-Juarez, F.d.R.; Galarza-Delgado, D.A. Adverse events of six COVID-19 vaccines in patients with autoimmune rheumatic diseases: A cross-sectional study. Rheumatol. Int. 2021, 41, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Botwin, G.J.; Li, D.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P.; Melmed, G.Y. Adverse Events after SARS-CoV-2 mRNA Vaccination Among Patients With Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 1746–1751. Available online: https://pubmed.ncbi.nlm.nih.gov/34047304 (accessed on 25 February 2022). [CrossRef] [PubMed]
- Lotan, I.; Wilf-Yarkoni, A.; Friedman, Y.; Stiebel-Kalish, H.; Steiner, I.; Hellmann, M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021, 28, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Chertcoff, A.; Leguizamón, F.d.V.; Goiry, L.G.; Eizaguirre, M.B.; Rodríguez, R.; Sosa, M.; Carballido, S.; Cruchet, V.; de Jong-Martis, A.; et al. Evaluation of short-term safety of COVID-19 vaccines in patients with multiple sclerosis from Latin America. Mult. Scler. J.-Exp. Transl. Clin. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Covidvax.live—Poland. Available online: https://covidvax.live/location/pol (accessed on 2 February 2022).
- Coronavirus Worldometers. 2022. Available online: https://www.worldometers.info/coronavirus (accessed on 2 February 2022).
- Wiysonge, C.S.; Ndwandwe, D.; Ryan, J.; Jaca, A.; Batouré, O.; Anya, B.P.M.; Cooper, S. Vaccine Hesitancy in the Era of COVID-19: Could Lessons from the Past Help in Divining the Future? Hum. Vaccin. Immunother. 2022, 18, 1–3. Available online: https://pubmed.ncbi.nlm.nih.gov/33684019 (accessed on 15 March 2022). [CrossRef] [PubMed]
- Wilson, S.L.; Wiysonge, C. Social Media and Vaccine Hesitancy. BMJ Glob. Health 2020, 5, e004206. Available online: https://gh.bmj.com/content/5/10/e0042062022 (accessed on 15 March 2022). [CrossRef] [PubMed]
| Demographics | No. | (%) | Range | Mean | MEDIAN | IQR | SD |
|---|---|---|---|---|---|---|---|
| Male | 667 | 29.5 | |||||
| Female | 1594 | 70.5 | |||||
| Male age | 19–71 | 41.8 | 41 | 16 | 10.8 | ||
| Female age | 18–74 | 43 | 43 | 16 | 11.4 | ||
| Clinical characteristics due to MS | |||||||
| Disease course | |||||||
| RRMS PPMS SPMS | 2149 52 60 | 95.1 2.3 2.7 | |||||
| EDSS | 0–8 | 2.4 | 2 | 2 | 1.45 | ||
| Disease duration (years) | 0–41 | 9.48 | 8 | 8 | 6.35 | ||
| Duration of DMT use (years) | 0–21 | 5.64 | 5 | 5 | 3.82 | ||
| The First Dose of COVID-19 Vaccination | The Second Dose of COVID-19 Vaccination | |||
|---|---|---|---|---|
| No. | [%] | No. | [%] | |
| Study population | 2261 | 2093 | ||
| Pain at the injection site | 1073 | 47.46 | 810 | 38.7 |
| Skin changes around the injection site | 106 | 4.69 | 85 | 4.06 |
| Fever, chills, flu-like symptoms | 387 | 17.12 | 428 | 20.45 |
| Fatigue | 233 | 10.31 | 236 | 11.28 |
| Headache | 206 | 9.11 | 198 | 9.46 |
| Muscle pain/Joint pain | 136 | 6.02 | 172 | 8.22 |
| Diarrhea | 2 | 0.09 | 2 | 0.1 |
| Nausea/Vomiting | 10 | 0.44 | 15 | 0.72 |
| Abdominal pain | 3 | 0.13 | 5 | 0.24 |
| Malaise | 133 | 5.88 | 172 | 8.22 |
| Anaphylaxis | 1 | 0.04 | 3 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnowska, A.; Tarasiuk, J.; Zajkowska, O.; Wnuk, M.; Marona, M.; Nowak, K.; Słowik, A.; Jamroz-Wiśniewska, A.; Rejdak, K.; Lech, B.; et al. Safety of Vaccines against SARS-CoV-2 among Polish Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines 2022, 10, 763. https://doi.org/10.3390/vaccines10050763
Czarnowska A, Tarasiuk J, Zajkowska O, Wnuk M, Marona M, Nowak K, Słowik A, Jamroz-Wiśniewska A, Rejdak K, Lech B, et al. Safety of Vaccines against SARS-CoV-2 among Polish Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines. 2022; 10(5):763. https://doi.org/10.3390/vaccines10050763
Chicago/Turabian StyleCzarnowska, Agata, Joanna Tarasiuk, Olga Zajkowska, Marcin Wnuk, Monika Marona, Klaudia Nowak, Agnieszka Słowik, Anna Jamroz-Wiśniewska, Konrad Rejdak, Beata Lech, and et al. 2022. "Safety of Vaccines against SARS-CoV-2 among Polish Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies" Vaccines 10, no. 5: 763. https://doi.org/10.3390/vaccines10050763
APA StyleCzarnowska, A., Tarasiuk, J., Zajkowska, O., Wnuk, M., Marona, M., Nowak, K., Słowik, A., Jamroz-Wiśniewska, A., Rejdak, K., Lech, B., Popiel, M., Rościszewska-Żukowska, I., Perenc, A., Bartosik-Psujek, H., Świderek-Matysiak, M., Siger, M., Ciach, A., Walczak, A., Jurewicz, A., ... Kułakowska, A. (2022). Safety of Vaccines against SARS-CoV-2 among Polish Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines, 10(5), 763. https://doi.org/10.3390/vaccines10050763









