Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Malaria Parasites and Blood-Stage Infection
2.3. Live Vaccination and Challenge Infection
2.4. Histopathology
2.5. Evaluation of Blood–Tissue Barrier
2.6. Antibody ELISA
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
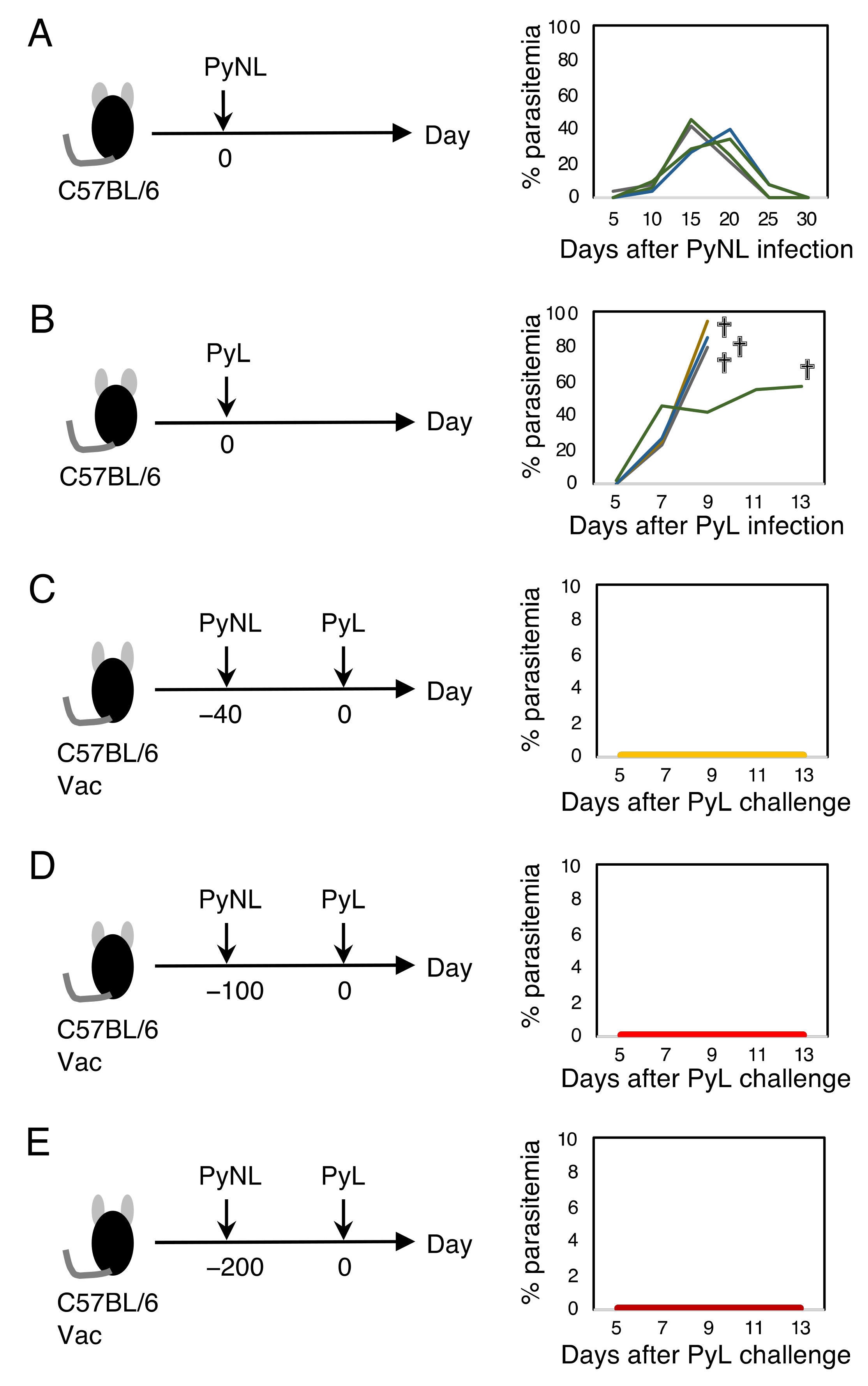
3.1. Live Vaccination Provided Complete Protection against Homologous Lethal Infection
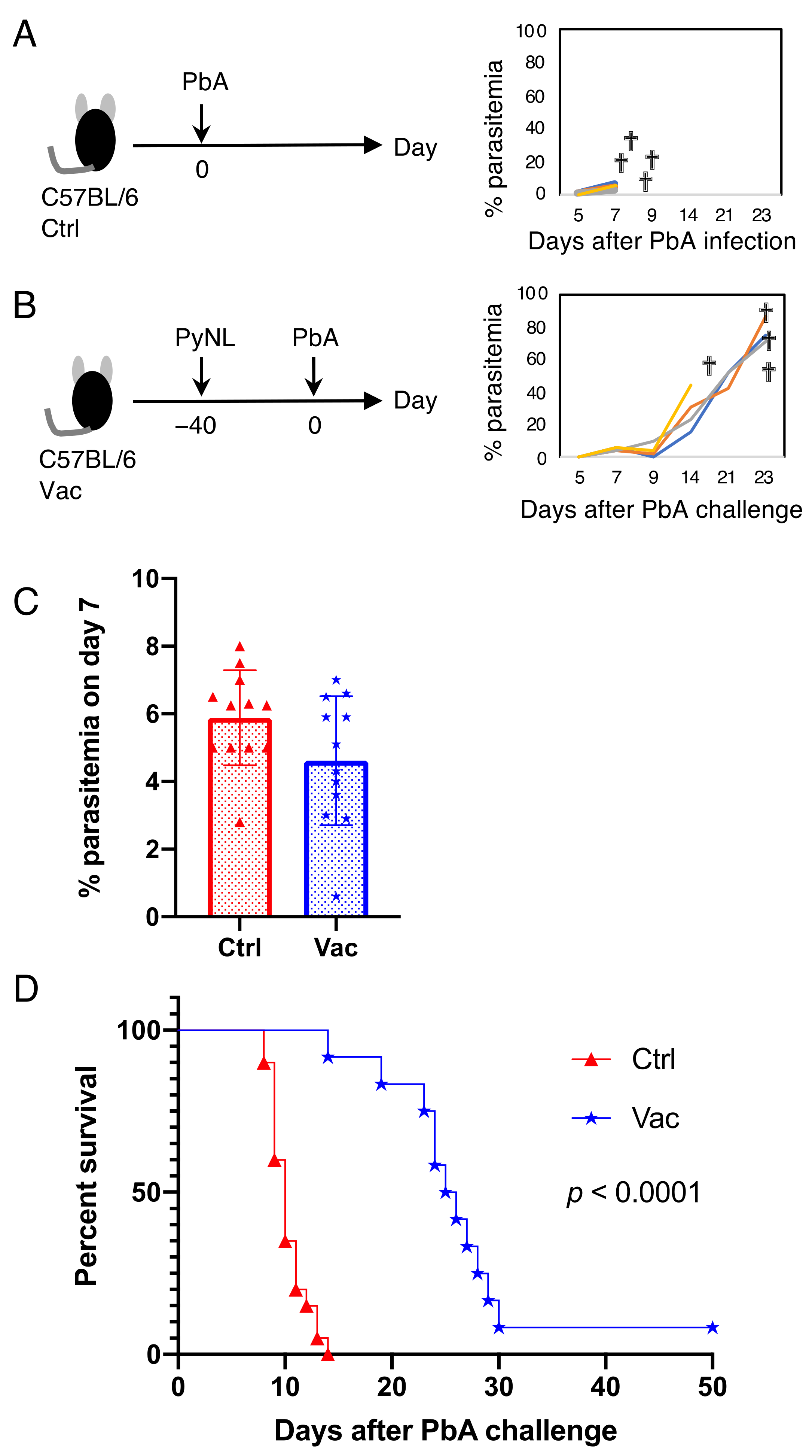
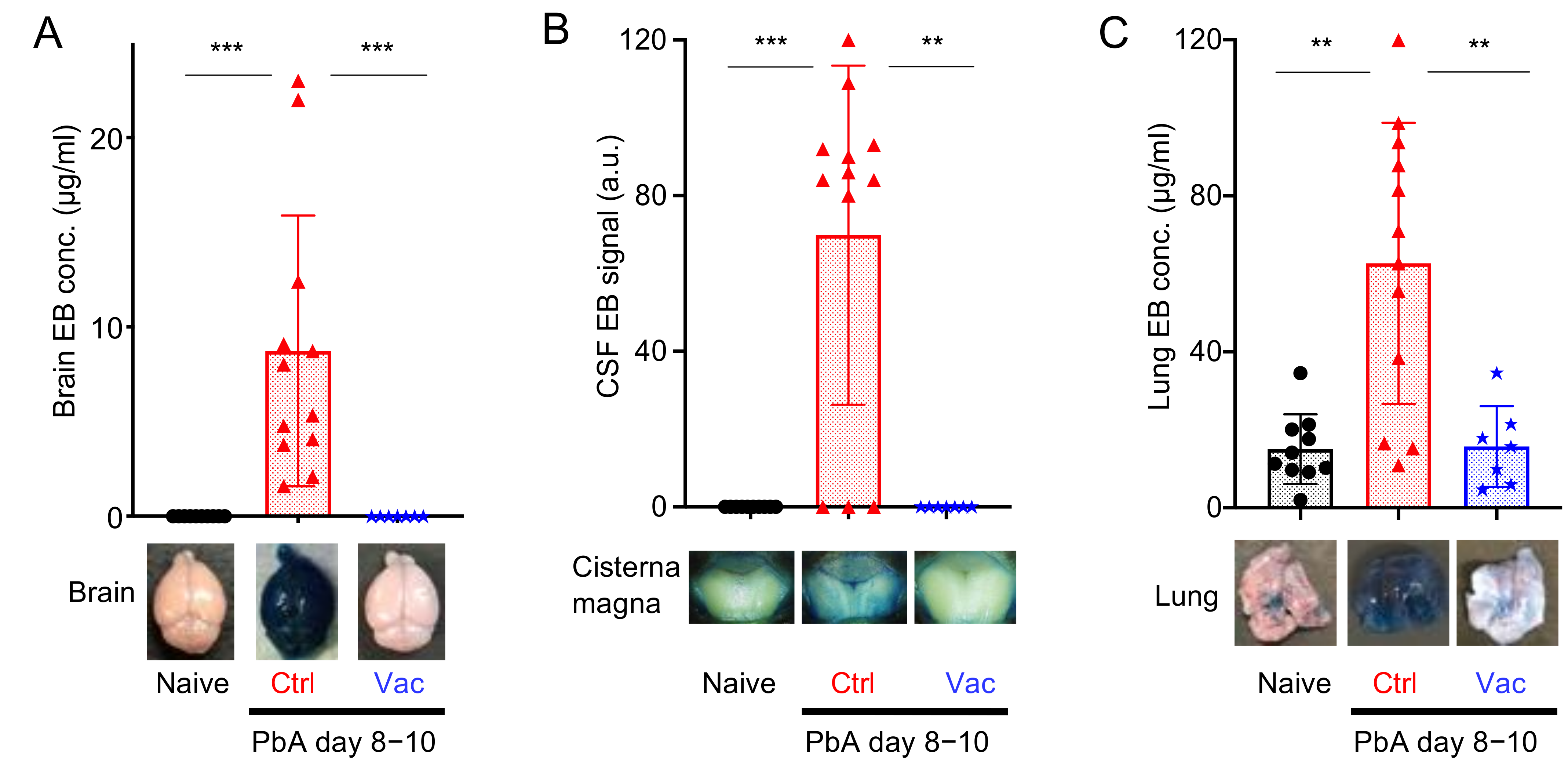
3.2. Live Vaccination Prevented the Development of ECM and Lung Pathology Following Heterologous Lethal Challenge
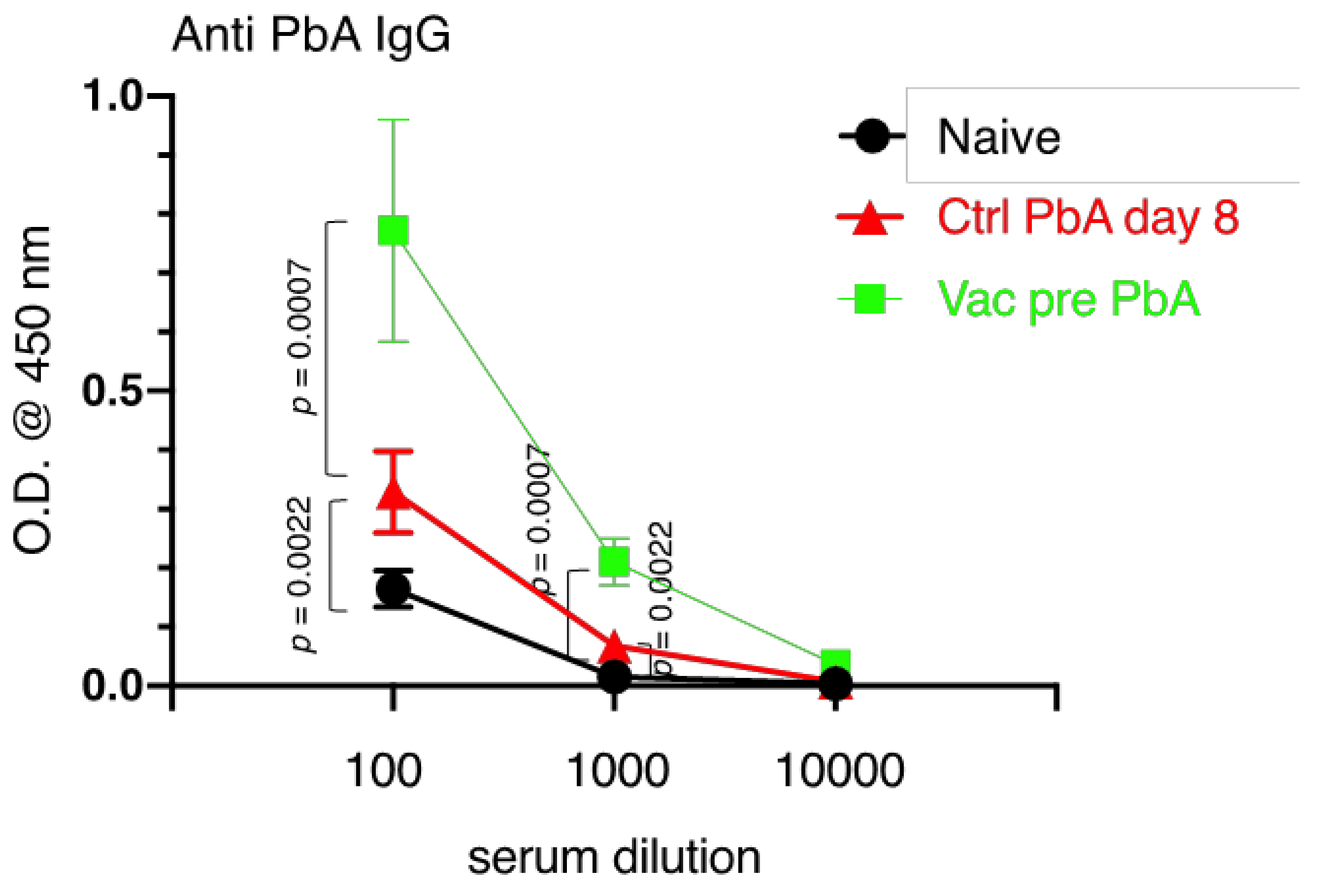
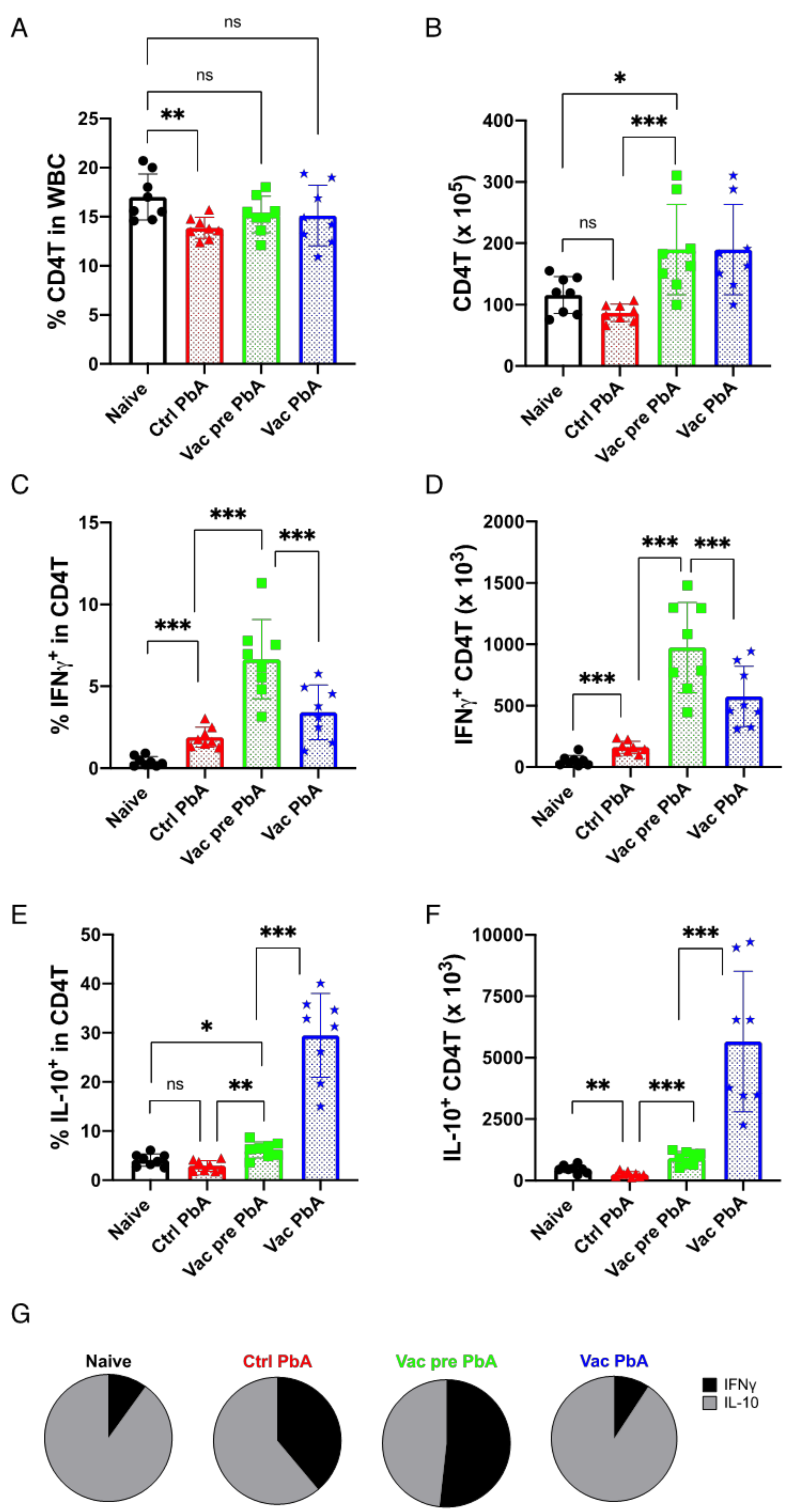
3.3. Immunological Characteristics of Live Vaccination with PyNL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.P.; Hwang, J.; Choi, L.; Lindblade, K.A.; Kachur, S.P.; Desai, M. Mass drug administration for malaria. Cochrane Database Syst. Rev. 2021, 9, CD008846. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Imwong, M.; Hien, T.T.; Thuy-Nhien, N.T.; Dondorp, A.M.; White, N.J. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect. Dis. 2017, 17, 1022–1023. [Google Scholar] [CrossRef]
- Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Artemisinin-Resistant Malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Matowo, N.S.; Martin, J.; Kulkarni, M.A.; Mosha, J.F.; Lukole, E.; Isaya, G.; Shirima, B.; Kaaya, R.; Moyes, C.; Hancock, P.A.; et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci. Rep. 2021, 11, 13457. [Google Scholar] [CrossRef]
- Bonam, S.R.; Renia, L.; Tadepalli, G.; Bayry, J.; Kumar, H.M.S. Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants. Vaccines 2021, 9, 1072. [Google Scholar] [CrossRef]
- Barfod, L.; Nielsen, M.A.; Turner, L.; Dahlback, M.; Jensen, A.T.; Hviid, L.; Theander, T.G.; Salanti, A. Baculovirus-expressed constructs induce immunoglobulin G that recognizes VAR2CSA on Plasmodium falciparum-infected erythrocytes. Infect. Immun. 2006, 74, 4357–4360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cotton, M. The Mosquirix (RTS.S) malaria vaccine. Trop. Doct. 2020, 50, 107. [Google Scholar] [CrossRef]
- Morrison, C. Landmark green light for Mosquirix malaria vaccine. Nat. Biotechnol. 2015, 33, 1015–1016. [Google Scholar] [CrossRef]
- Otieno, L.; Oneko, M.; Otieno, W.; Abuodha, J.; Owino, E.; Odero, C.; Mendoza, Y.G.; Andagalu, B.; Awino, N.; Ivinson, K.; et al. Safety and immunogenicity of RTS,S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: A randomised, double-blind, controlled trial. Lancet Infect. Dis. 2016, 16, 1134–1144. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Chandrudu, S.; Rigau-Planella, B.; Islam, M.T.; Cheong, Y.S.; Liu, G.; Wang, X.; Toth, I.; Hussein, W.M. Progress in the Development of Subunit Vaccines against Malaria. Vaccines 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jung, B.K.; Lee, J.J.; Pyo, K.H.; Kim, T.Y.; Choi, B.I.; Kim, T.W.; Hisaeda, H.; Himeno, K.; Shin, E.H.; et al. CD8 T-cell activation in mice injected with a plasmid DNA vaccine encoding AMA-1 of the reemerging Korean Plasmodium vivax. Korean J. Parasitol. 2011, 49, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Francica, J.R.; Shi, W.; Chuang, G.Y.; Chen, S.J.; Da Silva Pereira, L.; Farney, S.K.; Flynn, B.J.; Ou, L.; Stephens, T.; Tsybovsky, Y.; et al. Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria. Vaccines 2021, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Salman, A.M.; Cottingham, M.G.; Ewer, K.; Janse, C.J.; Khan, S.M.; Spencer, A.J.; Hill, A.V.S. Comparative assessment of vaccine vectors encoding ten malaria antigens identifies two protective liver-stage candidates. Sci. Rep. 2015, 5, 11820. [Google Scholar] [CrossRef] [PubMed]
- Jongo, S.A.; Urbano, V.; Church, L.W.P.; Olotu, A.; Manock, S.R.; Schindler, T.; Mtoro, A.; Kc, N.; Hamad, A.; Nyakarungu, E.; et al. Immunogenicity and Protective Efficacy of Radiation-Attenuated and Chemo-Attenuated PfSPZ Vaccines in Equatoguinean Adults. Am. J. Trop. Med. Hyg. 2021, 104, 283–293. [Google Scholar] [CrossRef]
- Mwakingwe-Omari, A.; Healy, S.A.; Lane, J.; Cook, D.M.; Kalhori, S.; Wyatt, C.; Kolluri, A.; Marte-Salcedo, O.; Imeru, A.; Nason, M.; et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 2021, 595, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, M.; Walk, J.; van der Boor, S.C.; Langenberg, M.C.C.; Hoogerwerf, M.A.; Janse, J.J.; Manurung, M.; Yap, X.Z.; Garcia, A.F.; Koopman, J.P.R.; et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci. Transl. Med. 2020, 12, eaaz5629. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Some, A.; Traore, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Murphy, S.C.; Deye, G.A.; Sim, B.K.L.; Galbiati, S.; Kennedy, J.K.; Cohen, K.W.; Chakravarty, S.; Kc, N.; Abebe, Y.; James, E.R.; et al. PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: A randomized, placebo-controlled trial with controlled human malaria infection. PLoS Pathog. 2021, 17, e1009594. [Google Scholar] [CrossRef]
- Van Schaijk, B.C.; Ploemen, I.H.; Annoura, T.; Vos, M.W.; Foquet, L.; van Gemert, G.J.; Chevalley-Maurel, S.; van de Vegte-Bolmer, M.; Sajid, M.; Franetich, J.F.; et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. eLife 2014, 3, e03582. [Google Scholar] [CrossRef]
- Lyke, K.E.; Ishizuka, A.S.; Berry, A.A.; Chakravarty, S.; DeZure, A.; Enama, M.E.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; Manoj, A.; et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc. Natl. Acad. Sci. USA 2017, 114, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Shen, J.; Chou, B.; Duan, X.; Tu, L.; Tetsutani, K.; Moriya, C.; Ishida, H.; Hamano, S.; Shimokawa, C.; et al. Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur. J. Immunol. 2010, 40, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ishida, H.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Suzuki, T.; Shimokawa, C.; Hisaeda, H. CD8 T cell activation by murine erythroblasts infected with malaria parasites. Sci. Rep. 2013, 3, 1572. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Iwawaki, T.; Akai, R.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Hisaeda, H. Evaluating experimental cerebral malaria using oxidative stress indicator OKD48 mice. Int. J. Parasitol. 2014, 44, 681–685. [Google Scholar] [CrossRef]
- Imai, T.; Ishida, H.; Suzue, K.; Taniguchi, T.; Okada, H.; Shimokawa, C.; Hisaeda, H. Cytotoxic activities of CD8 T cells collaborate with macrophages to protect against blood-stage murine malaria. eLife 2015, 4, e04232. [Google Scholar] [CrossRef]
- Imai, T.; Suzue, K.; Ngo-Thanh, H.; Ono, S.; Orita, W.; Suzuki, H.; Shimokawa, C.; Olia, A.; Obi, S.; Taniguchi, T.; et al. Fluctuations of Spleen Cytokine and Blood Lactate, Importance of Cellular Immunity in Host Defense Against Blood Stage Malaria Plasmodium yoelii. Front. Immunol. 2019, 10, 2207. [Google Scholar] [CrossRef]
- Imai, T.; Suzue, K.; Ngo-Thanh, H.; Shimokawa, C.; Hisaeda, H. Potential and Limitations of Cross-Protective Vaccine against Malaria by Blood-Stage Naturally Attenuated Parasite. Vaccines 2020, 8, 375. [Google Scholar] [CrossRef]
- Ngo-Thanh, H.; Sasaki, T.; Suzue, K.; Yokoo, H.; Isoda, K.; Kamitani, W.; Shimokawa, C.; Hisaeda, H.; Imai, T. Blood-cerebrospinal fluid barrier: Another site disrupted during experimental cerebral malaria caused by Plasmodium berghei ANKA. Int. J. Parasitol. 2020, 50, 1167–1175. [Google Scholar] [CrossRef]
- Belnoue, E.; Kayibanda, M.; Vigario, A.M.; Deschemin, J.C.; van Rooijen, N.; Viguier, M.; Snounou, G.; Renia, L. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J. Immunol. 2002, 169, 6369–6375. [Google Scholar] [CrossRef]
- Renia, L.; Grau, G.E.; Wassmer, S.C. CD8+ T cells and human cerebral malaria: A shifting episteme. J. Clin. Invest. 2020, 130, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Yui, K. Cross-presentation of malaria antigen by brain microvessels: Why CD8(+) T cells are critical for the pathogenesis of experimental cerebral malaria. EMBO Mol. Med. 2013, 5, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, C.; van de Wiel, T.; Mommers, E.; Sauerwein, R.; Eling, W. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 1997, 114 Pt 1, 7–12. [Google Scholar] [CrossRef]
- Beghdadi, W.; Porcherie, A.; Schneider, B.S.; Dubayle, D.; Peronet, R.; Huerre, M.; Watanabe, T.; Ohtsu, H.; Louis, J.; Mecheri, S. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J. Exp. Med. 2008, 205, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Evans, K.J.; Schofield, L.; Davenport, M.P. Preferential invasion of reticulocytes during late-stage Plasmodium berghei infection accounts for reduced circulating reticulocyte levels. Int. J. Parasitol. 2006, 36, 1389–1397. [Google Scholar] [CrossRef]
- Junqueira, C.; Barbosa, C.R.R.; Costa, P.A.C.; Teixeira-Carvalho, A.; Castro, G.; Sen Santara, S.; Barbosa, R.P.; Dotiwala, F.; Pereira, D.B.; Antonelli, L.R.; et al. Cytotoxic CD8(+) T cells recognize and kill Plasmodium vivax-infected reticulocytes. Nat. Med. 2018, 24, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foa, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Van der Horst, H.J.; Nijhof, I.S.; Mutis, T.; Chamuleau, M.E.D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers 2020, 12, 3041. [Google Scholar] [CrossRef]
- Naing, C.; Whittaker, M.A.; Nyunt Wai, V.; Mak, J.W. Is Plasmodium vivax malaria a severe malaria?: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3071. [Google Scholar] [CrossRef]
- Lovegrove, F.E.; Gharib, S.A.; Pena-Castillo, L.; Patel, S.N.; Ruzinski, J.T.; Hughes, T.R.; Liles, W.C.; Kain, K.C. Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model. PLoS Pathog. 2008, 4, e1000068. [Google Scholar] [CrossRef]
- Ghosh, D.; Stumhofer, J.S. The spleen: “Epicenter” in malaria infection and immunity. J. Leukoc. Biol. 2021, 110, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Yoeli, M.; Hargreaves, B.; Carter, R.; Walliker, D. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann. Trop. Med. Parasitol. 1975, 69, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sedegah, M.; Weiss, W.W.; Hoffman, S.L. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol. 2007, 29, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Reiman, J.M.; Rodriguez, I.B.; Ito, K.; Yanow, S.K.; El-Deeb, I.M.; Batzloff, M.R.; Stanisic, D.I.; Engwerda, C.; Spithill, T.; et al. Cross-species malaria immunity induced by chemically attenuated parasites. J. Clin. Investig. 2013, 123, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Marin-Mogollon, C.; Imai, T.; Mendes, A.M.; van der Laak, R.; Sturm, A.; Geurten, F.J.A.; Miyazaki, S.; Chevalley-Maurel, S.; Ramesar, J.; et al. Generation of a Genetically Modified Chimeric Plasmodium falciparum Parasite Expressing Plasmodium vivax Circumsporozoite Protein for Malaria Vaccine Development. Front. Cell. Infect. Microbiol. 2020, 10, 591046. [Google Scholar] [CrossRef]
- Aikawa, M.; Iseki, M.; Barnwell, J.W.; Taylor, D.; Oo, M.M.; Howard, R.J. The pathology of human cerebral malaria. Am. J. Trop. Med. Hyg. 1990, 43, 30–37. [Google Scholar] [CrossRef]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- Langhorne, J.; Quin, S.J.; Sanni, L.A. Mouse models of blood-stage malaria infections: Immune responses and cytokines involved in protection and pathology. Chem. Immunol. 2002, 80, 204–228. [Google Scholar] [CrossRef]
- Matsuzaki-Moriya, C.; Tu, L.; Ishida, H.; Imai, T.; Suzue, K.; Hirai, M.; Tetsutani, K.; Hamano, S.; Shimokawa, C.; Hisaeda, H. A critical role for phagocytosis in resistance to malaria in iron-deficient mice. Eur. J. Immunol. 2011, 41, 1365–1375. [Google Scholar] [CrossRef]
- Duan, X.; Imai, T.; Chou, B.; Tu, L.; Himeno, K.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Shimokawa, C.; et al. Resistance to Malaria by Enhanced Phagocytosis of Erythrocytes in LMP7-deficient Mice. PLoS ONE 2013, 8, e59633. [Google Scholar] [CrossRef][Green Version]
- Cockburn, I.A.; Seder, R.A. Malaria prevention: From immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 2018, 19, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Aitken, E.H.; Mahanty, S.; Rogerson, S.J. Antibody effector functions in malaria and other parasitic diseases: A few needles and many haystacks. Immunol. Cell Biol. 2020, 98, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Doolan, D.L. Malaria vaccine design: Immunological considerations. Immunity 2010, 33, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef]
- Leitner, W.W.; Haraway, M.; Pierson, T.; Bergmann-Leitner, E.S. Role of Opsonophagocytosis in Immune Protection against Malaria. Vaccines 2020, 8, 264. [Google Scholar] [CrossRef]
- Grau, G.E.; Heremans, H.; Piguet, P.F.; Pointaire, P.; Lambert, P.H.; Billiau, A.; Vassalli, P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1989, 86, 5572–5574. [Google Scholar] [CrossRef]
- Kumar, R.; Ng, S.; Engwerda, C. The Role of IL-10 in Malaria: A Double Edged Sword. Front. Immunol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Specht, S.; Ruiz, D.F.; Dubben, B.; Deininger, S.; Hoerauf, A. Filaria-induced IL-10 suppresses murine cerebral malaria. Microbes Infect. 2010, 12, 635–642. [Google Scholar] [CrossRef]
- Niikura, M.; Kamiya, S.; Nakane, A.; Kita, K.; Kobayashi, F. IL-10 plays a crucial role for the protection of experimental cerebral malaria by co-infection with non-lethal malaria parasites. Int. J. Parasitol. 2010, 40, 101–108. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Geurts, N.; Deroost, K.; Van Aelst, I.; Verhenne, S.; Heremans, H.; Van Damme, J.; Opdenakker, G. Immunopathology and dexamethasone therapy in a new model for malaria-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care. Med. 2010, 181, 957–968. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Gerrard, J.; Fink, J.; Griffin, P.M.; Liu, X.Q.; Sundac, L.; Sekuloski, S.; Rodriguez, I.B.; Pingnet, J.; Yang, Y.; et al. Infectivity of Plasmodium falciparum in Malaria-Naive Individuals Is Related to Knob Expression and Cytoadherence of the Parasite. Infect. Immun. 2016, 84, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Ackerman, H.C.; Su, X.Z.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Staalsoe, T.; Lavstsen, T.; Jensen, A.T.; Sowa, M.P.; Arnot, D.E.; Hviid, L.; Theander, T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003, 49, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gangnard, S.; Round, A.; Dechavanne, S.; Juillerat, A.; Raynal, B.; Faure, G.; Baron, B.; Ramboarina, S.; Singh, S.K.; et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. USA 2010, 107, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.; Sekuloski, S.; Odedra, A.; Woolley, S.; Jennings, H.; Amante, F.; Trenholme, K.R.; Healer, J.; Cowman, A.F.; Eriksson, E.M.; et al. Safety, infectivity and immunogenicity of a genetically attenuated blood-stage malaria vaccine. BMC Med. 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Witschkowski, J.; Behrends, J.; Frank, R.; Eggers, L.; von Borstel, L.; Hertz, D.; Mueller, A.K.; Schneider, B.E. BCG Provides Short-Term Protection from Experimental Cerebral Malaria in Mice. Vaccines 2020, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Strangward, P.; Haley, M.J.; Shaw, T.N.; Schwartz, J.M.; Greig, R.; Mironov, A.; de Souza, J.B.; Cruickshank, S.M.; Craig, A.G.; Milner, D.A., Jr.; et al. A quantitative brain map of experimental cerebral malaria pathology. PLoS Pathog. 2017, 13, e1006267. [Google Scholar] [CrossRef]
- Barrera, V.; Haley, M.J.; Strangward, P.; Attree, E.; Kamiza, S.; Seydel, K.B.; Taylor, T.E.; Milner, D.A., Jr.; Craig, A.G.; Couper, K.N. Comparison of CD8(+) T Cell Accumulation in the Brain During Human and Murine Cerebral Malaria. Front. Immunol. 2019, 10, 1747. [Google Scholar] [CrossRef]
- Riggle, B.A.; Manglani, M.; Maric, D.; Johnson, K.R.; Lee, M.H.; Neto, O.L.A.; Taylor, T.E.; Seydel, K.B.; Nath, A.; Miller, L.H.; et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J. Clin. Investig. 2020, 130, 1128–1138. [Google Scholar] [CrossRef]
- Van Eijk, L.E.; Binkhorst, M.; Bourgonje, A.R.; Offringa, A.K.; Mulder, D.J.; Bos, E.M.; Kolundzic, N.; Abdulle, A.E.; van der Voort, P.H.; Olde Rikkert, M.G.; et al. COVID-19: Immunopathology, pathophysiological mechanisms, and treatment options. J. Pathol. 2021, 254, 307–331. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care. Med. 2021, 203, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.G.; Grau, G.E.; Janse, C.; Kazura, J.W.; Milner, D.; Barnwell, J.W.; Turner, G.; Langhorne, J.; Participants of the Hinxton Retreat meeting on Animal Models for Research on Severe, M. The role of animal models for research on severe malaria. PLoS Pathog. 2012, 8, e1002401. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, T.; Ngo-Thanh, H.; Suzue, K.; Shimo, A.; Nakamura, A.; Horiuchi, Y.; Hisaeda, H.; Murakami, T. Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria. Vaccines 2022, 10, 762. https://doi.org/10.3390/vaccines10050762
Imai T, Ngo-Thanh H, Suzue K, Shimo A, Nakamura A, Horiuchi Y, Hisaeda H, Murakami T. Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria. Vaccines. 2022; 10(5):762. https://doi.org/10.3390/vaccines10050762
Chicago/Turabian StyleImai, Takashi, Ha Ngo-Thanh, Kazutomo Suzue, Aoi Shimo, Akihiro Nakamura, Yutaka Horiuchi, Hajime Hisaeda, and Takashi Murakami. 2022. "Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria" Vaccines 10, no. 5: 762. https://doi.org/10.3390/vaccines10050762
APA StyleImai, T., Ngo-Thanh, H., Suzue, K., Shimo, A., Nakamura, A., Horiuchi, Y., Hisaeda, H., & Murakami, T. (2022). Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria. Vaccines, 10(5), 762. https://doi.org/10.3390/vaccines10050762






