Abstract
The Vaccine Adverse Event Reporting System database has been used to report adverse events following several vaccines. We studied the patient population predisposed to such reactions and how these reactions differ with respect to the vaccine type. We searched the electronic databases PubMed, EMBASE, and Scopus up to 9 July 2021 for any study describing cardiac adverse events attributed to the vaccination. A total of 56 studies met the criteria comprising 340 patients. There were 20 studies describing cardiac adverse events following smallpox vaccination, 11 studies describing adverse events after influenza vaccination, and 18 studies describing adverse events after COVID-19 vaccination. There was a total of six studies describing cardiac adverse events after the pneumococcal vaccine, tetanus toxoid, cholera vaccine, and rabies vaccine. Adverse events following influenza vaccination occurred more commonly in older females within an average duration of four days from vaccination. Pericardial involvement was the most reported adverse event. Adverse events following COVID-19 vaccination happened at a mean age of 42.7 years, more commonly in males, and mostly after a second dose. Adverse events following smallpox vaccination occurred more commonly in younger males, with an average onset of symptoms from vaccination around 16.6 days. Adverse events were mostly myopericarditis; however, the acute coronary syndrome has been reported with some vaccines.
1. Introduction
Vaccination has remained an integral part of primary care medicine for preventing common and life-threatening diseases for decades. Vaccination has been associated with minor injection site reactions, fever, fatigue, and lymphadenopathy; however, serious neurological and cardiac adverse events (AEs) have been known to occur [1]. The Vaccine Adverse Event Reporting System (VAERS), a passive surveillance database, provides information on reports of AEs after vaccination with approved vaccines in the United States (2). Through this passive reporting, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) conduct post-licensure vaccine safety monitoring [2]. A study on the VAERS database from 1990 to 2018 showed 0.1% (708) myopericarditis cases out of the 620,195 reports of possible adverse events to VAERS [3]. At the end of 2019, a novel coronavirus now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the cause of pneumonia cases in Wuhan, China [4]. It rapidly spread, resulting in a global pandemic affecting 400 million people worldwide and has taken approximately 6 million lives till now. In order to fight this infection, there was an emergent authorization of several vaccines by the World Health Organization all over the world. Two of them are Coronavirus disease (COVID-19) mRNA vaccines: BNT162b2 (Pfizer-BioNTech COVID-19 vaccine) and mRNA-1273 (Moderna COVID-19 vaccine), three are adenoviral vector vaccines: Ad26.COV2.S (Janssen COVID-19 vaccine, also referred to as the Johnson & Johnson vaccine) and AZD1222(Oxford/AstraZeneca), Covishield (Oxford/AstraZeneca), and lastly, three are inactivated vaccines: Covaxin (Bharat Biotech), BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac) [5]. Soon after, various reports of adverse events (AEs) from the COVID-19 vaccines emerged [6,7,8,9]. The aim of our review is to investigate the patient characteristics and provide an overview of the management for patients that develop AEs after vaccination.
2. Methods
2.1. Design
We conducted a systematic review with a priori selection and outcome criteria according to “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” guidelines. We registered the study protocol in Prospero with CRD42021267467. We searched the electronic databases PubMed, EMBASE, and Scopus. The search included articles published from inception up to 9 July 2021. The MeSH (Medical Subject Headings) search terms were “myocarditis” or “myocardium” or “pericardial” or “pericardium” or “acute myocardial injury” OR “acute coronary syndrome” OR “heart failure” OR “arrhythmia” OR “troponin” OR “ischemia” OR “acute myocardial infarction” OR “coronary events” OR “creatine kinase” OR “heart” AND “vaccine”.
We excluded studies involving patients below 18 years of age. We included a study if it described cardiac AEs associated with the vaccination. We excluded any animal studies. Our primary outcome was to see the cardiac AEs reported to any vaccination.
2.2. Study Selection
Two authors (KP and GDRP) independently reviewed the retrieved abstracts and assessed eligibility. The full-text review was conducted when either of the reviewers of the abstracts felt that the citations might meet inclusion criteria. Disagreement was resolved by the engagement of a third author (SS).
2.3. Data Extraction
Data from the included studies were independently extracted by three authors (KP, GDRP, SS). We extracted the following data: age, gender, comorbidities, vaccine type, vaccine dose, number of days after which the event happened, inflammatory markers, imaging, results of an endomyocardial biopsy, and treatment instituted.
3. Results
Overall, 7327 studies were found on the PubMed, Embase, and Scopus databases (Figure 1). After removing duplicates, 6095 studies were screened by title and abstract for any cardiac AEs after vaccination. A total of 267 studies qualified and were opened for a full-text review. A final selection of 56 studies was selected for adverse cardiac events after vaccination, with a total of 340 patients. Eleven studies described cardiac AEs following influenza vaccination [10,11,12,13,14,15,16,17,18,19,20], and 18 studies described cardiac AEs after COVID-19 vaccination [6,7,8,9,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. There were 20 studies reporting cardiac AEs after smallpox vaccination [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Among other vaccines, there were two reports of cardiac AEs after pneumococcal vaccination [56,57], three reports of cardiac AEs after tetanus toxoid [58,59,60], one report following cholera vaccination [61], and one report following rabies vaccine [62].
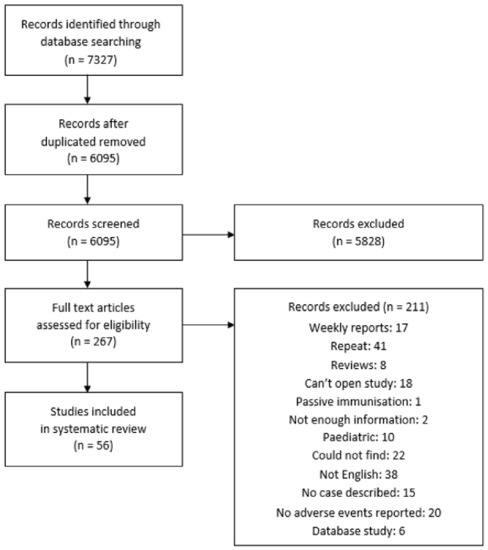
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow diagram.
The median age for developing cardiac AEs following vaccination was 43.79 ± 21.2 years. Most of the patients described were males (84%) and rest were females (Table 1).

Table 1.
Summary of Vaccine Related Cardiac Adverse Events.
A total of 34 patients were included in cardiac AEs following influenza vaccination, with the mean age of patients being 68.55 ± 18.23 years. Fifty-five percent of cases described were female. Myocarditis/pericarditis/myopericarditis developed in 29 patients, and Takotsubo cardiomyopathy was described in two cases. The average time of symptom onset from vaccination was 4.7 ± 4 days. Anti-inflammatory treatment was used in 66% of patients, and one patient required an extracorporeal membrane oxygenator. Steroids were used on one patient. All patients recovered (Table 2).

Table 2.
Adverse events after Influenza vaccination.
A total of 67 patients developed cardiac AEs following COVID-19 vaccination. Overall, 35 cases reported AEs after BNT162b2 (Pfizer), 26 cases developed cardiac AEs after mRNA-1273, four cases developed cardiac AEs after AZD1222, and two cases developed cardiac AEs after receiving CoronaVac and JNJ-78436735. The average age for these patients was 42.7 years (SD = 19.6), with 59 (88%) patients being male gender. Most patients (69.6%) developed a reaction after the second dose. Myocarditis/pericarditis/myopericarditis developed in 35 patients. Acute coronary syndrome (ACS) was described in six patients. There was one case of myocardial infarction (MI) with non-obstructive coronary arteries (MINOCA) and one stress-induced cardiomyopathy. The average time of symptom onset from vaccination was 2.34 days (SD = 1.83 days). Moreover, 15 cases received anti-inflammatory treatment, and the same number of patients received colchicine. All patients recovered except one (Table 3).

Table 3.
Adverse events after COVID-19 vaccination.
A total of 232 developed adverse events following smallpox vaccination with a mean age of 29.48 ± 8.9 years. Most of these AEs (82.75%) were described in male patients. Most patients (212) developed myocarditis/myopericarditis or pericarditis. Twenty-six patients had ACS, and there were two cases of arrhythmias. The average duration of symptom onset from the time of vaccination was 16.6 days (SD = 14). For treatment, nine cases described the use of NSAIDs, and three patients received colchicine. Most patients developed AE following the first dose, but 30 patients developed a reaction after the second dose. There were three cases with mortality. Endomyocardial biopsy (EMB)was performed in eight cases, and four cases had eosinophilic infiltration (Table 4).

Table 4.
Adverse events after Smallpox vaccination.
Cardiac AEs were also described with the pneumococcal vaccine, mostly in the elderly age group. Myopericarditis was also reported with tetanus toxoid in three males with an average age of 25 years. All three patients recovered, and a biopsy in one case showed eosinophilic infiltration. There was also one case of ACS reported after a cholera vaccination and myocarditis after a rabies vaccination (Table 5).

Table 5.
Adverse events after other vaccinations.
4. Discussion
Myocarditis is characterized by inflammation of the heart, and in resource-abundant countries, viral infections are the most frequently presumed cause of myocarditis [63]. Often pericarditis and myocarditis are observed in tandem; hence the term myopericarditis being recognized by the European Society of Cardiology (ESC). The annual incidence of myocarditis in the United States is estimated to be 1 to 10 per 100,000 of the population [44]. Vaccine-associated myocarditis is a rare event that was recognized as an adverse event after the mass revaccination against the smallpox virus began in military personnel. The new guidelines by ESC have now recognized inflammatory cardiomyopathy into four groups based on EMB results: inflammation-negative, virus-negative; inflammation-positive, virus-negative; inflammation-negative, virus-positive; and inflammation-positive, virus-positive [64]. Vaccine-associated cardiomyopathy usually falls into the virus-negative category. Characteristics of predisposed patients and treatment strategies remain to be defined.
The incidence of myopericarditis rose from 0.08 per 1000 to 0.11 per 1000 after the resumption of smallpox vaccination in 2002 [37]. Recent vaccination against the deadly COVID-19 virus has raised similar concerns of myopericarditis with an absolute rate of 1.7 per 1,000,000 vaccinated individuals as described by Husby et al. in a Denmark-based cohort study. However, these rates are much lower than the incidence rate described for viral myocarditis (10 to 22 per 100,000 individuals) [65]. Thus, there should be no vaccine hesitancy based on these cardiac AEs; however, physicians should be cautious about the development of these AEs, and care should not be delayed if suspicion arises.
Su et al.’s study on the VAERS database from 1990 to 2018 showed that most patients developing myopericarditis from vaccination were 19–49 years old, and 90% of this age group were male with symptom onset 8–14 days after vaccination [3]. The vaccines frequently associated with these AEs in order of frequency were smallpox, anthrax, typhoid, and inactivated influenza. Cardiac AEs from hepatitis B, zoster vaccine, hepatitis A, varicella, hemophilus, influenza, polio, and pneumococcal vaccine were also reported. We tried to study how these reactions differ with respect to the type of vaccine.
AEs following smallpox vaccination are the most studied among all vaccines. A prospective study by Engler et al. in military personnel saw an increased incidence of new-onset cardiac symptoms following smallpox and trivalent inactivated influenza with a relative risk difference of 16.11 between vaccinated and non-vaccinated populations [37]. Thus, vaccinia-associated inflammatory disease was defined as any cardiac inflammatory syndrome occurring within 30 days of vaccination without another identifiable cause [66]. Reif et al. described that the hyperactivation of inflammatory response from the variola virus in the smallpox vaccine is responsible for these AEs following smallpox vaccination. The study identified increased monocyte recruitment followed by upregulation of intercellular adhesion molecule 1 in patients developing adverse events. The activated macrophages then produce cytokine interleukin-10 (IL-10), which along with certain genotypes of IL-4, leads to increased production of granulocyte stimulating factor-3, a cytokine produced by activated T cells, macrophages, and endothelial cells to increase production of neutrophils for inflammatory reactions [67].
Influenza vaccination has been associated with a decrease in all-cause mortality in heart failure patients [68]. A literature search in 2017 described seven cases of pericarditis in patients above 60 years of age following influenza vaccination, as was seen in our analysis [12]. In a case series of 84 pericarditis cases by Zanettini et al., 23 cases were thought to be due to influenza vaccination in elderly females [18]. The mean time of symptom onset was seven days, as was seen in our analysis [12]. The mechanisms of systemic immunologic reactivity for pericarditis remain to be proven because of its rarity. Following influenza vaccination, there is a systemic inflammatory reaction, and it is postulated that the AEs, particularly TTC, may be due to this increased sympathetic discharge [15].
The first reports of AEs following COVID-19 vaccination came from Israel’s Health Ministry, which reported heart inflammation in cases who received the Pfizer vaccine [69]. Soon after, a CDC advisory committee on immunization practices identified a likely association between the COVID-19 mRNA vaccines and cases of myocarditis and pericarditis [70]. Based on data from the VAERS, the CDC has estimated that the incidence of myocarditis after COVID-19 vaccination is 0.48 cases per 100,000 overall and 1.2 cases per 100,000 among vaccine recipients between the ages of 18 and 29 years. However, based on the study by Witberg et al. on the Israel database incidence of myocarditis, it was estimated that there were 2.13 cases per 100,000 vaccinated persons in the 42 days after the first vaccine dose [71]. It is important to consider these case reports within the broader context of the COVID-19 pandemic, which has caused tremendous morbidity and mortality throughout the world.
COVID-19 vaccination, especially AstraZeneca, was also found to be temporally related to thrombosis, but causality could not be proved; hence the vaccine was suspended [26,72]. Myocardial infarction (MI), in particular, is one of the most dreaded cardiac complications, as was seen in our included studies. The initial data from clinical trials by FDA briefing documents demonstrated that the incidence of MI was 0.02% and 0.03%, respectively, in the vaccine group. Later, a study on an elderly age group, comparing vaccinated and unvaccinated patients, did not show any significant increase in any cardiovascular events such as stroke or pulmonary embolisms [73].
As described earlier, these cardiac AEs are rare and different mechanisms have been proposed for these reactions, including molecular mimicry between immunogens in vaccines and human cells [74,75]. There is also the possibility of interaction between the encoded viral spike protein, antibodies generated by the host, and a yet undetermined cardiac protein in susceptible hosts [75]. Although these mechanisms for AEs are still enigmatic, a preponderance for male gender and younger age was observed in the VAERS database and our review [76]. The association of myocarditis with male sex and younger age could be attributed to sex hormones which may account for a more intense inflammatory response [77]. As suggested by experimental studies on myocarditis in mice, testosterone may be implicated in the inhibition of anti-inflammatory cells and the stimulation of immune responses by mRNA vaccine [78]. The presentation of symptoms within approximately two days of receiving a second dose (68% of patients) of mRNA vaccination also suggests an immune-mediated reaction in the host. Furthermore, cases of Type 1 Kounis syndrome have been described after inactivated COVID-19 vaccine, indicating an allergic reaction to a vaccine component [32].
According to 2012 ESC, cardiac magnetic resonance imaging (CMR) is the noninvasive gold standard method for the diagnosis of myocarditis [65]. CMR findings, including regional dysfunction, late gadolinium enhancement, and elevated native T1 and T2, have been used in many of these cases for myocarditis diagnosis following COVID-19 vaccination [7,9,22]. EMB, which is the gold standard, was performed on five patients with myocarditis after smallpox vaccination and in one patient after COVID-19 vaccination [65]. Three of them showed eosinophilic infiltrate. Yamamoto et al. also described biopsy-proven eosinophilic myocarditis following tetanus toxoid, which responded to high-dose corticosteroid treatment [59].
Specific guidelines for the management of vaccinia-associated myopericarditis have been outlined by the Department of Defense Vaccine Healthcare Center, and symptomatic patients should receive treatment with analgesics and/or NSAIDs as a first-line treatment [66]. Most cases in our study received colchicine and ibuprofen. In patients with persistent symptoms, steroids were advised [66]. The ESC guidelines advise immunosuppressive therapy for virus-negative myocarditis; however, the data are still unclear [79]. It is recommended that in patients refractory to standard therapy with no contraindications, treatment must be tailored on an individual basis [79]. Steroids were used for many patients in this review, which led to improvement.
5. Limitations
The review could not include all studies from the VAERS database. It was limited to full-text articles describing the patient and the cardiac AEs. The review also mostly comprised of case reports, case series, and retrospective studies with few prospective studies. Therefore, there is a potential risk of bias, and the results should be interpreted with some caution. Given the rarity of these events and the retrospective nature of the events, it is not possible to estimate the relative risk of these AEs. Lastly, most of the studies did not provide all the required information, particularly about the results of cardiac testing and management.
6. Conclusions
Although vaccination remains a pivotal pillar of our healthcare to fight against some of the deadliest infections, understanding vaccine-associated cardiac adverse events will improve our healthcare delivery to this subpopulation. The incidence of cardiac AEs from vaccination remains much lower than cardiac AEs from other causes, but providers should be cautious of these AEs after vaccination.
Author Contributions
Drafting of the manuscript: K.P., S.S., G.D.R.-P.; Concept and design: K.P., G.D.R.-P.; Acquisition, analysis, or interpretation of data: K.P., S.S., G.D.R.-P.; Critical revision of the manuscript for important intellectual content: E.A.-S., P.S.; Supervision: E.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No IRB approval required.
Conflicts of Interest
The authors declare no conflict of interest.
References
- VAERS. Vaccine Adverse Event Reporting System. Available online: https://vaers.hhs.gov/about.html (accessed on 15 January 2022).
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; McNeil, M.M.; Welsh, K.J.; Marquez, P.L.; Ng, C.; Yan, M.; Cano, M.V. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine 2021, 39, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, R.; Kaur, J.; Pandey, S.; Sharma, V.; Thakur, L.; Sati, S.; Mani, S.; Asthana, S.; Sharma, T.K.; et al. Wuhan to World: The COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2021, 11, 596201. [Google Scholar] [CrossRef] [PubMed]
- Team MCVT. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 24 November 2021).
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196. [Google Scholar] [CrossRef]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T., Jr.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef]
- Mansour, J.; Short, R.G.; Bhalla, S.; Woodard, P.K.; Verma, A.; Robinson, X.; Raptis, D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: A report of two cases. Clin. Imaging 2021, 78, 247–249. [Google Scholar] [CrossRef]
- Kao, C.-D.; Chen, J.-T.; Lin, K.-P.; Shan, D.-E.; Wu, Z.-A.; Liao, K.-K. Guillain-Barré syndrome coexisting with pericarditis or nephrotic syndrome after influenza vaccination. Clin. Neurol. Neurosurg. 2004, 106, 136–138. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bae, J.I.; Ryoo, S.M.; Kim, W.Y. Acute Fulminant Myocarditis Following Influenza Vaccination Requiring Extracorporeal Membrane Oxygenation. Acute Crit. Care 2019, 34, 165–169. [Google Scholar] [CrossRef]
- Mei, R.; Raschi, E.; Poluzzi, E.; Diemberger, I.; De Ponti, F. Recurrence of pericarditis after influenza vaccination: A case report and review of the literature. BMC Pharmacol. Toxicol. 2018, 19, 20. [Google Scholar] [CrossRef]
- De Meester, A.; Luwaert, R.; Chaudron, J.M. Symptomatic pericarditis after influenza vaccination: Report of two cases. Chest 2000, 117, 1803–1805. [Google Scholar] [CrossRef][Green Version]
- Santoro, F.; Ieva, R.; Ferraretti, A.; Carpagnano, G.; Pappalardo, I.; De Gennaro, L.; Di Biase, M.; Brunetti, N.D. Tako-Tsubo cardiomyopathy after influenza vaccination. Int. J. Cardiol. 2013, 167, e51–e52. [Google Scholar] [CrossRef]
- Singh, K.; Marinelli, T.; Horowitz, J.D. Takotsubo cardiomyopathy after anti-influenza vaccination: Catecholaminergic effects of immune system. Am. J. Emerg. Med. 2013, 31, e1–e4. [Google Scholar] [CrossRef]
- Streifler, J.J.; Dux, S.; Garty, M.; Rosenfeld, J.B. Recurrent pericarditis: A rare complication of influenza vaccination. Br. Med. J. 1981, 283, 526–527. [Google Scholar] [CrossRef][Green Version]
- Cheng, M.P.; Kozoriz, M.G.; Ahmadi, A.A.; Kelsall, J.; Paquette, K.; Onrot, J.M. Post-vaccination myositis and myocarditis in a previously healthy male. Allergy Asthma Clin. Immunol. 2016, 12, 6. [Google Scholar] [CrossRef]
- Zanettini, M.T.; Zanettini, J.O.; Zanettini, J.P. Pericarditis. Series of 84 consecutive cases. Arq. Bras. Cardiol. 2004, 82, 360–369. [Google Scholar] [CrossRef][Green Version]
- Godreuil, S.; Delhaume, O.; Besset-Prat, L.; Blayac, J.P.; Peyriere, H.; Bonnet, P. Acute haemorrhagic pericarditis following influenza vaccination. Presse Med. 2003, 32, 258–259. [Google Scholar]
- Wiley. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/all.12252 (accessed on 24 November 2021).
- Lee, E.; Chew, N.W.S.; Ng, P.; Yeo, T.J. Reply to “Letter to the editor: Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following PfizerBioNTech COVID-19 vaccination”. QJM 2021, 114, hcab231. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202. [Google Scholar] [CrossRef]
- Lee, E.; Chew, N.W.S.; Ng, P.; Yeo, T.J. A spectrum of cardiac manifestations post Pfizer-BioNTech COVID-19 vaccination. QJM 2021, 114, 661–662. [Google Scholar] [CrossRef]
- Habib, M.B.; Hamamyh, T.; Elyas, A.; Altermanini, M.; Elhassan, M. Acute myocarditis following administration of BNT162b2 vaccine. IDCases 2021, 25, e01197. [Google Scholar] [CrossRef]
- Mouch, S.A.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ojha, U.K.; Vardhan, B.; Tiwari, A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab. Syndr. 2021, 15, 1055–1056. [Google Scholar] [CrossRef]
- Deb, A.; Abdelmalek, J.; Iwuji, K.; Nugent, K. Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report and Review of Current Evidence from Vaccine Adverse Events Reporting System Database. J. Prim. Care Community Health 2021, 12, 21501327211029230. [Google Scholar] [CrossRef]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cattafi, A.; Carerj, M.L.; Booz, C.; Ascenti, G.; Cicero, G.; Blandino, A.; Mazziotti, S. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can. J. Cardiol. 2021, 37, 1665–1667. [Google Scholar] [CrossRef]
- Watkins, K.; Griffin, G.; Septaric, K.; Simon, E.L. Myocarditis after BNT162b2 vaccination in a healthy male. Am. J. Emerg. Med. 2021, 50, 815.e1–815.e2. [Google Scholar] [CrossRef]
- Ammirati, E.; Cavalotti, C.; Milazzo, A.; Pedrotti, P.; Soriano, F.; Schroeder, J.W.; Morici, N.; Giannattasio, C.; Frigerio, M.; Metra, M.; et al. Temporal Relation Between Second Dose BNT162b2 mRNA Covid-19 Vaccine and Cardiac involvement in a Patient with Previous SARS-COV-2 Infection. Int. J. Cardiol. Heart Vasc. 2021, vol.34, 100774. [Google Scholar]
- Tajstra, M.; Jaroszewicz, J.; Gąsior, M. Acute Coronary Tree Thrombosis After Vaccination for COVID-19. JACC Cardiovasc. Interv. 2021, 14, e103–e104. [Google Scholar] [CrossRef]
- Srinivasan, K.N.; Sathyamurthy, I.; Neelagandan, M. Relation between COVID-19 vaccination and myocardial infarction—Casual or coincidental? IHJ Cardiovasc. Case Rep. 2021, 5, 71–74. [Google Scholar] [CrossRef]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis Syndrome Induced by Inactivated SARS-COV-2 Vaccine. J. Emerg. Med. 2021, 61, e71–e76. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Timothy, J.; Whitman, M.; Ferguson, A.; Catherine, F.; Decker, A. Cardiac Dysrhythmia following Smallpox Vaccination. Clin. Infect. Dis. 2003, 37, 1579–1580. [Google Scholar] [CrossRef][Green Version]
- Engler, R.J.; Nelson, M.R.; Collins, L.C., Jr.; Spooner, C.; Hemann, B.A.; Gibbs, B.T.; Atwood, J.E.; Howard, R.S.; Chang, A.S.; Cruser, D.L.; et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE. 2015, 10, e0118283. [Google Scholar] [CrossRef] [PubMed]
- Eckart, R.E.; Love, S.S.; Atwood, J.E.; Arness, M.K.; Cassimatis, D.C.; Campbell, C.L.; Boyd, S.Y.; Murphy, J.G.; Swerdlow, D.L.; Collins, L.C.; et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 2004, 44, 201–205. [Google Scholar] [CrossRef]
- Sarkisian, S.A.; Hand, G.; Rivera, V.M.; Smith, M.; Miller, J.A. A Case Series of Smallpox Vaccination-Associated Myopericarditis: Effects on Safety and Readiness of the Active Duty Soldier. Mil. Med. 2019, 184, e280–e283. [Google Scholar] [CrossRef]
- Saurina, G.; Shirazi, S.; Lane, J.M.; Daniel, B.; Di Eugenia, L. Myocarditis after Smallpox Vaccination: A Case Report. Clin. Infect. Dis. 2003, 37, 145–146. [Google Scholar] [CrossRef]
- Sharma, U.; Tak, T. A report of 2 cases of myopericarditis after Vaccinia virus (smallpox) immunization. WMJ 2011, 110, 291–294. [Google Scholar]
- Sniadack, M.M.; Neff, L.J.; Swerdlow, D.L.; Schieber, R.A.; McCauley, M.M.; Mootrey, G.T. Follow-Up of Cardiovascular Adverse Events after Smallpox Vaccination among Civilians in the United States, 2003. Clin. Infect. Dis. 2008, 46, S251–S257. [Google Scholar] [CrossRef][Green Version]
- Keinath, K.; Church, T.; Kurth, B.; Hulten, E. Myocarditis secondary to smallpox vaccination. BMJ Case Rep. 2018, 2018, bcr–2017–223523. [Google Scholar] [CrossRef]
- Halsell, J.S.; Riddle, J.R.; Atwood, J.E.; Gardner, P.; Shope, R.; Poland, G.A.; Gray, G.C.; Ostroff, S.; Eckart, R.E.; Hospenthal, D.R.; et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003, 289, 3283–3289. [Google Scholar] [CrossRef]
- Matthews, A.W.; Griffiths, I.D. Post-vaccinial pericarditis and myocarditis. Br. Heart J. 1974, 36, 1043–1045. [Google Scholar] [CrossRef]
- Eckart, R.E.; Shry, E.A.; Jones SOt Atwood, J.E.; Grabenstein, J.D. Comparison of clinical presentation of acute myocarditis following smallpox vaccination to acute coronary syndromes in patients <40 years of age. Am. J. Cardiol. 2005, 95, 1252–1255. [Google Scholar]
- Cangemi, V.F. Acute pericarditis after smallpox vaccination. N. Engl. J. Med. 1958, 258, 1257–1259. [Google Scholar] [CrossRef]
- Taylor, C.L.; Eckart, R.E. Chest pain, ST elevation, and positive cardiac enzymes in an austere environment: Differentiating smallpox vaccination-mediated myocarditis and acute coronary syndrome in Operation Iraqi Freedom. J. Emerg. Med. 2012, 42, 267–270. [Google Scholar] [CrossRef]
- Murphy, J.G.; Wright, R.S.; Bruce, G.K.; Baddour, L.M.; Farrell, A.M.; Edwards, W.D.; Kita, H.; Cooper, L.T. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet 2003, 362, 1378–1380. [Google Scholar] [CrossRef]
- Guerdan, B.R.; Shumway, G.J. Case Report: A Presumptive Case of Vaccinia Myocarditis. Mil. Med. 2004, 169, 866–867. [Google Scholar] [CrossRef][Green Version]
- Docekal, J.W.; Francisco, G.; Lee, J.C. Recurrent epicardial ventricular tachycardia following smallpox vaccination. Heart Rhythm Case Rep. 2018, 5, 6–9. [Google Scholar] [CrossRef]
- Finlay-Jones, L.R. Fatal myocarditis after vaccination against smallpox—Report of a case. N. Engl. J. Med. 1964, 270, 41–42. [Google Scholar] [CrossRef]
- Dalgaard, J.B. Fatal myocarditis following smallpox vaccination. Am. Heart J. 1957, 54, 156–157. [Google Scholar] [CrossRef]
- Bruner, D.I.; Butler, B.S. Smallpox vaccination-associated myopericarditis is more common with the newest smallpox vaccine. J. Emerg. Med. 2014, 46, e85–e87. [Google Scholar] [CrossRef]
- Lin, A.H.; Phan, H.A.; Barthel, R.V.; Maisel, A.S.; Crum-Cianflone, N.F.; Maves, R.C.; Nayak, K.R. Myopericarditis and pericarditis in the deployed military member: A retrospective series. Mil. Med. 2013, 178, 18–20. [Google Scholar] [CrossRef]
- Makaryus, A.N.; Revere, D.J.; Steinberg, B. Recurrent reversible dilated cardiomyopathy secondary to viral and streptococcal pneumonia vaccine-associated myocarditis. Cardiol. Rev. 2006, 14, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, P.; Gertner, E.; McEvoy, C.E. Severe polyserositis induced by the 13-valent pneumococcal conjugate vaccine: A case report. J. Med. Case Rep. 2017, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Boccara, F.; Benhaiem-Sigaux, N.; Cohen, A. Acute myopericarditis after diphtheria, tetanus, and polio vaccination. Chest 2001, 120, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Hashimoto, T.; Ohta-Ogo, K.; Ishibashi-Ueda, H.; Imanaka-Yoshida, K.; Hiroe, M.; Yokochi, T. A case of biopsy-proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc. Pathol. 2018, 37, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Boroujerdi-Rad, L. Acute myopericarditis after tetanus, diptheria, and pertussis vaccination in a healthy adult. J. Gen. Intern. Med. 2014, 29, S298. [Google Scholar]
- Koutsaimanis, K.G.; Rée, G.H. Possible association of myocardial infarction and active immunization. N. Engl. J. Med. 1978, 299, 153–154. [Google Scholar]
- Karger. Available online: https://wwwkargercom/Article/Pdf/167026 (accessed on 15 December 2021).
- Seferović, P.M.; Tsutsui, H.; McNamara, D.M.; Ristić, A.D.; Basso, C.; Bozkurt, B.; Jr, L.T.C.; Filippatos, G.; Ide, T.; Inomata, T.; et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur. J. Hear. Fail. 2021, 23, 854–871. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1591–1601. [Google Scholar] [CrossRef]
- Cassimatis, D.C.; Atwood, J.E.; Engler, R.M.; Linz, P.E.; Grabenstein, J.D.; Vernalis, M.N. Smallpox vaccination and myopericarditis: A clinical review. J. Am. Coll. Cardiol. 2004, 43, 1503–1510. [Google Scholar] [CrossRef]
- Reif, D.M.; Motsinger-Reif, A.A.; McKinney, B.A.; Rock, M.T.; Crowe, J.E.; Moore, J.H. Integrated analysis of genetic and proteomic data identifies biomarkers associated with adverse events following smallpox vaccination. Genes Immun. 2009, 10, 112–119. [Google Scholar] [CrossRef]
- Rodrigues, B.S.; Alves, M.; Duarte, G.S.; Costa, J.; Pinto, F.J.; Caldeira, D. The impact of influenza vaccination in patients with cardiovascular disease: An overview of systematic reviews. Trends Cardiovasc. Med. 2021, 31, 315–320. [Google Scholar] [CrossRef]
- Reuters. Israel Examining Heart Inflammation Cases in People Who Received Pfizer COVID Shot. Available online: https://www.reuters.com/world/middle-east/israel-examining-heart-inflammation-cases-people-who-received-pfizer-covid-shot-2021-04-25/ (accessed on 15 January 2022).
- Hannah, G.; Rosenblum, M. Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices—United States, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1094. [Google Scholar]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Bardaweel, S.K.; Tropsha, A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines 2021, 9, 1186. [Google Scholar] [CrossRef]
- Lazaros, G.; Klein, A.L.; Hatziantoniou, S.; Tsioufis, C.; Tsakris, A.; Anastassopoulou, C. The Novel Platform of mRNA COVID-19 Vaccines and Myocarditis: Clues into the Potential Underlying Mechanism. Vaccine 2021, 39, 4925–4927. [Google Scholar] [CrossRef]
- Huber, S.A.; Pfaeffle, B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 1994, 68, 5126–5132. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).