A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Antigens
2.2. Vaccine Immunization in Animals
2.3. Antigen-Specific Antibody ELISA Assay
2.4. SARS-CoV-2 Pseudovirus-Based Neutralization Assay
2.5. Detection of Th1 and Th2 Cytokines
2.6. Statistical Analysis
3. Results
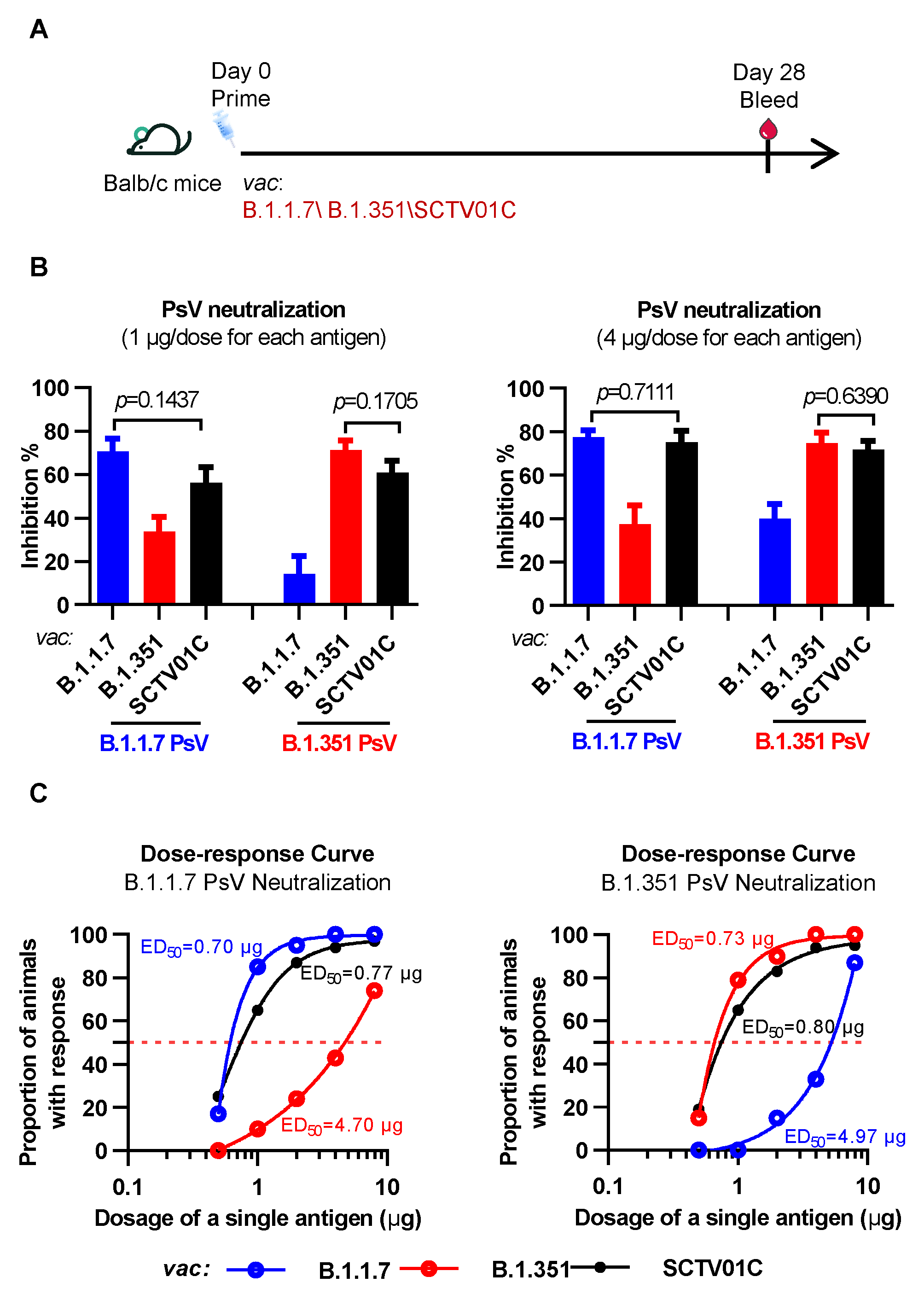
3.1. Humoral Immune Responses Induced by the B.1.1.7 Monovalent, B.1.351 Monovalent and SCTV01C Bivalent Vaccines after a Single Immunization in Mice
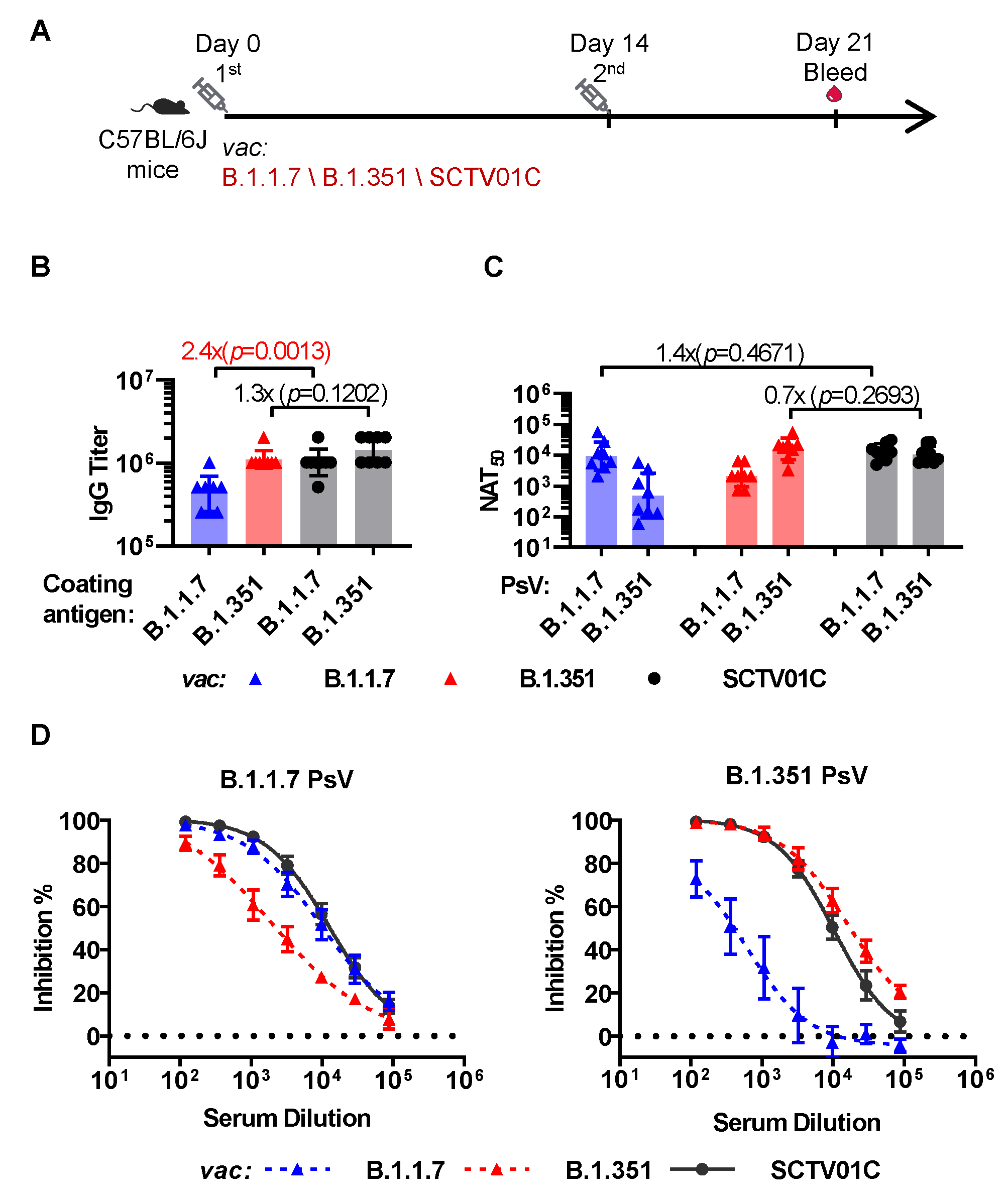
3.2. Humoral Immune Responses Induced by the B.1.1.7 Monovalent, B.1.351 Monovalent and SCTV01C Bivalent Vaccines with a Prime-Boost Regimen in Mice
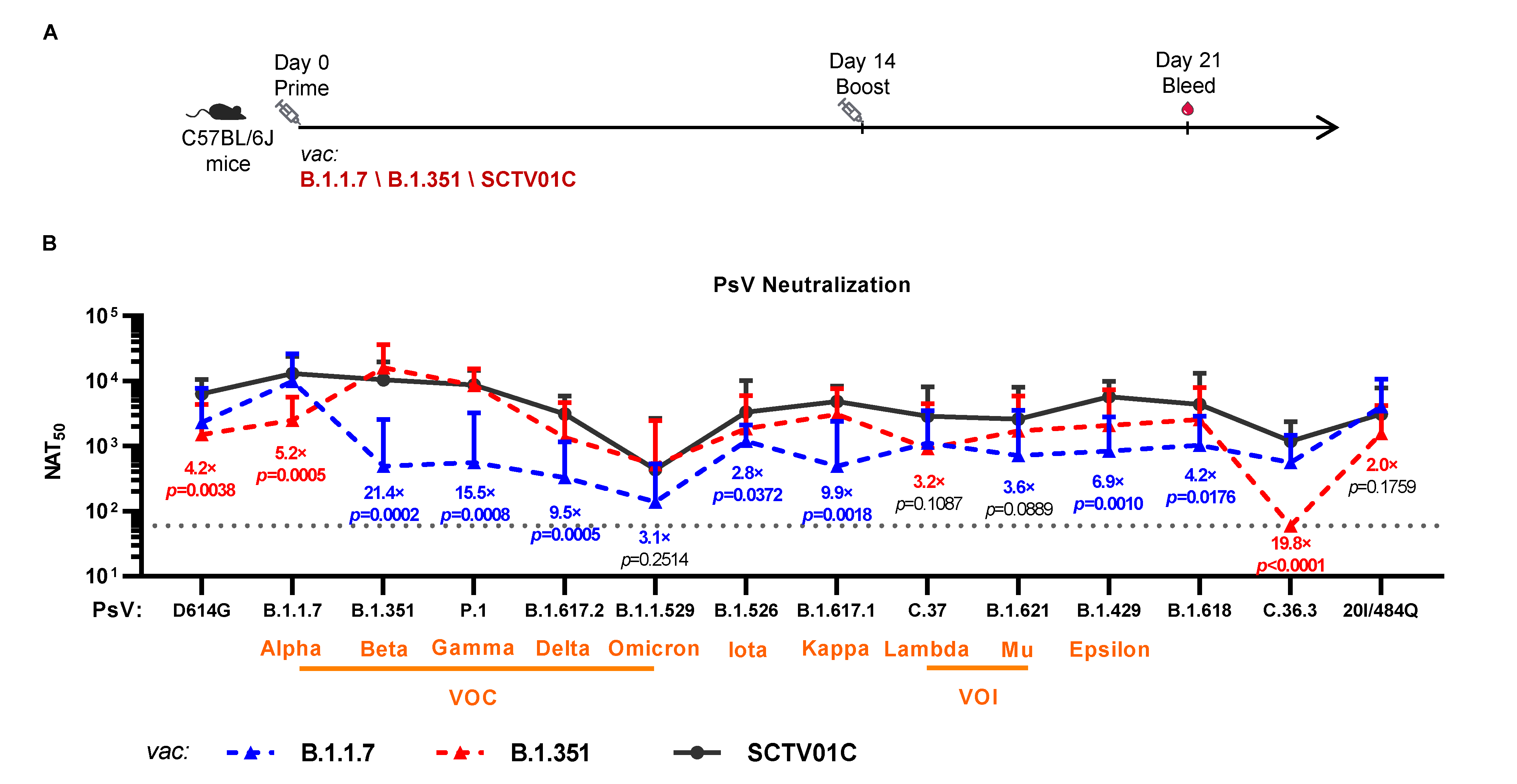
3.3. Cross-Neutralizing Activities Elicited by the B.1.1.7 Monovalent, B.1.351 Monovalent and SCTV01C Bivalent Vaccines
| Vaccines | Variants | D614G | VOC | VOI | VUM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B.1 | B.1.1.7 | B.1.351 | P.1 | B.1.617.2 | B.1.1.529 | C.37 | B.1.621 | B.1.526 | B.1.617.1 | B.1.429 | C.36.3 | B.1.618 | 20I/484Q | ||
| / | (Alpha) | (Beta) | (Gamma) | (Delta) | (Omicron) | (Lambda) | (Mu) | (Iota) | (Kappa) | (Epsilon) | / | / | / | ||
| B.1.1.7 Vaccine | NAT50 | 2323 | 9412 | 489 | 563 | 328 | 139 | 1114 | 720 | 1194 | 490 | 840 | 563 | 1027 | 4040 |
| Fold Reduction a | 4.1 | 1.0 | 19.2 | 16.7 | 28.7 | 67.8 | 8.4 | 13.1 | 7.9 | 19.2 | 11.2 | 16.7 | 9.2 | 2.3 | |
| B.1.351 Vaccine | NAT50 | 1511 | 2131 | 16,106 | 8644 | 1364 | 519 | 913 | 1726 | 1848 | 3071 | 2073 | 60 c | 2570 | 1574 |
| Fold Reduction b | 10.7 | 7.6 | 1.0 | 1.9 | 11.8 | 18.1 | 17.6 | 9.3 | 8.7 | 5.2 | 7.8 | 268.4 | 6.3 | 10.2 | |
| SCTV01C Bivalent Vaccine | NAT50 | 6411 | 13,019 | 10,480 | 8745 | 3134 | 437 | 2892 | 2595 | 3324 | 4846 | 5761 | 1185 | 4365 | 3134 |
| Fold Increase (vs B.1.1.7 vac) | 2.8 | 1.4 | 21.4 | 15.5 | 9.6 | 3.1 | 2.6 | 3.6 | 2.8 | 9.9 | 6.9 | 2.1 | 4.2 | 0.8 | |
| Fold Increase (vs B.1.351 vac) | 4.2 | 6.1 | 0.7 | 1 | 2.3 | 0.8 | 3.2 | 1.5 | 1.8 | 1.6 | 2.8 | 19.8 | 1.7 | 2 | |
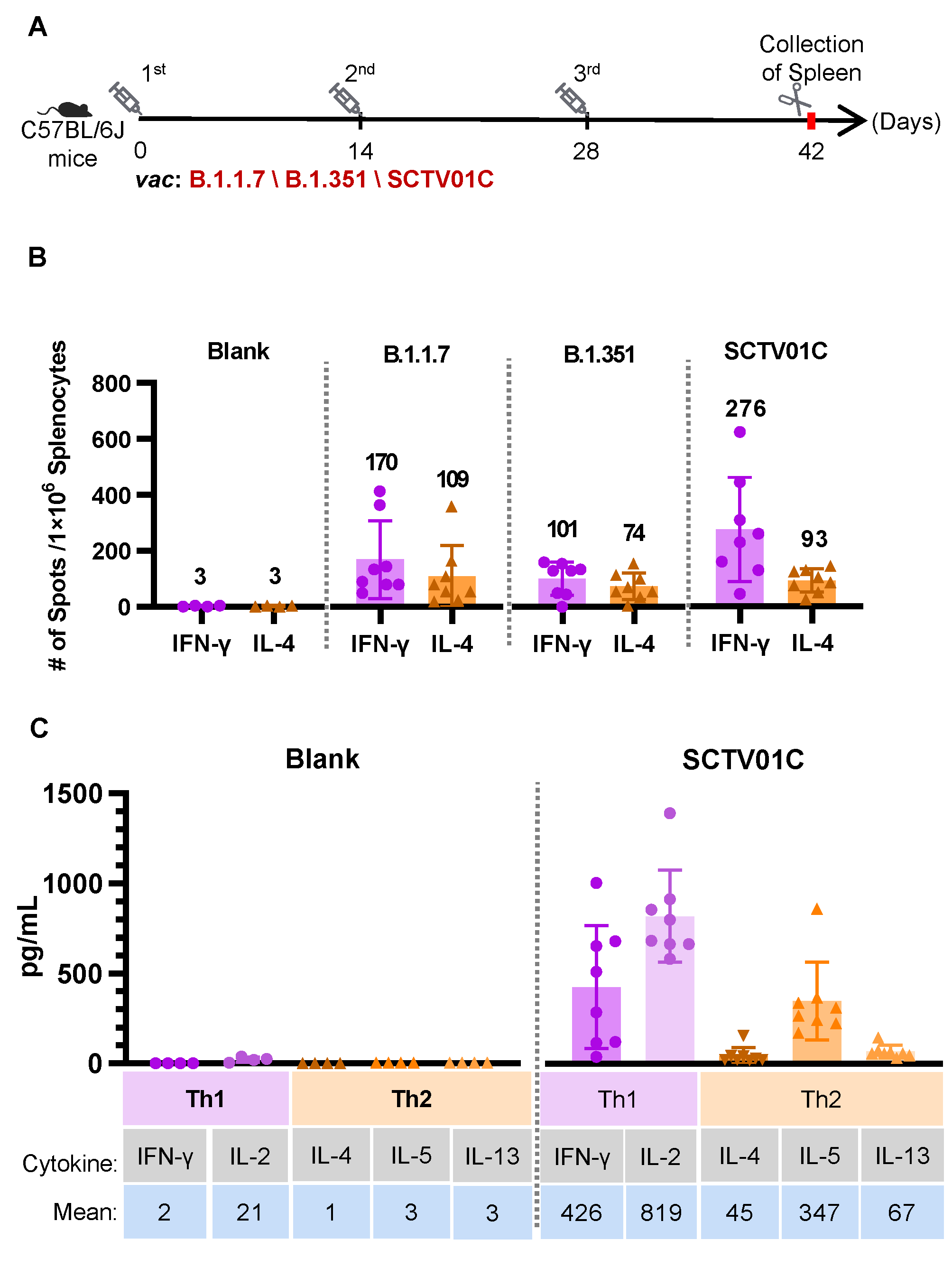
3.4. T Cell Responses Induced by the B.1.1.7 Monovalent, B.1.351 Monovalent and SCTV01C Bivalent Vaccines
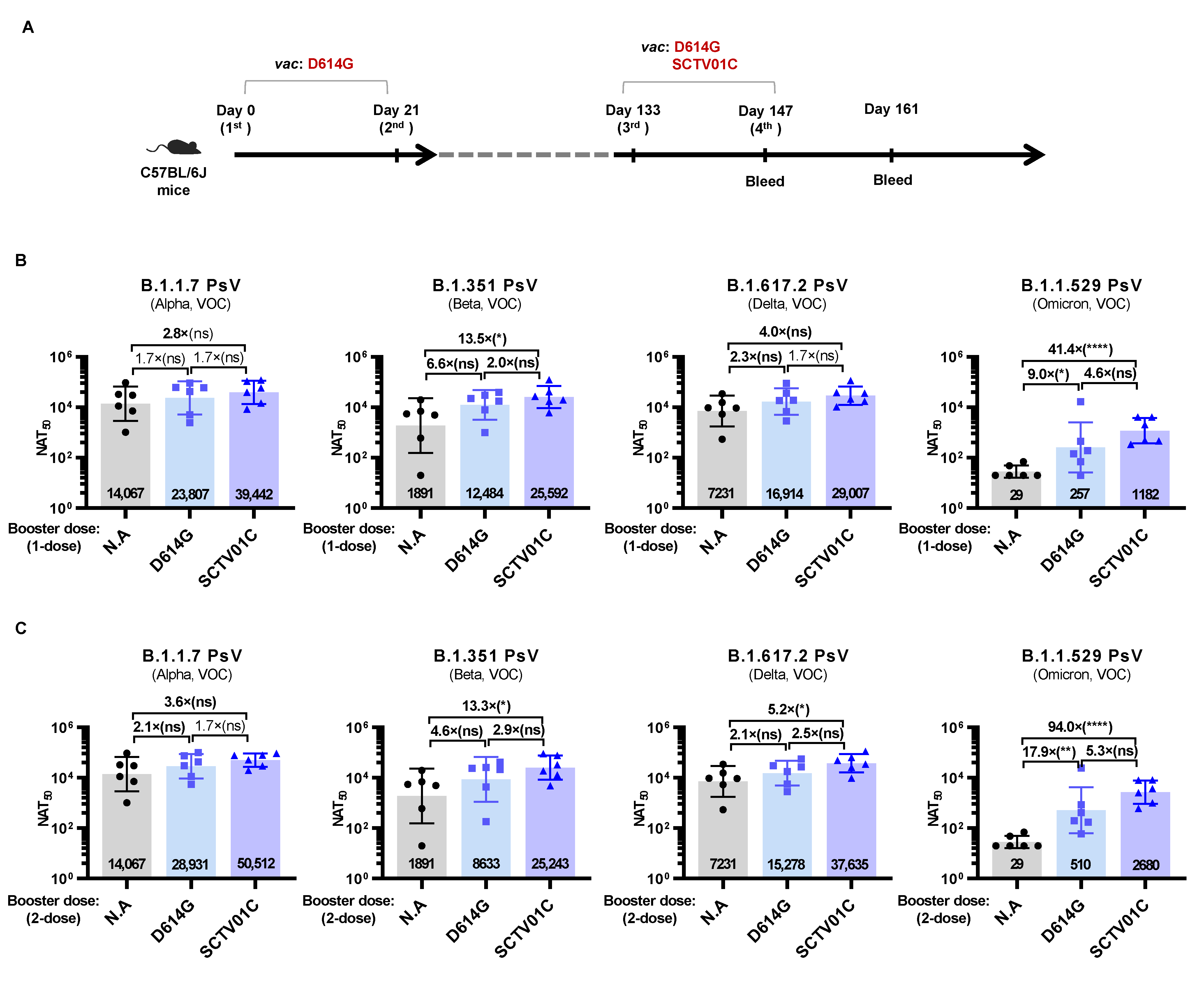
3.5. Humoral Immune Responses Induced by Booster Dose of SCTV01C
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 26 January 2021).
- Shao, Y.; Wu, Y.; Feng, Y.; Xu, W.; Xiong, F.; Zhang, X. SARS-CoV-2 vaccine research and immunization strategies for improved control of the COVID-19 pandemic. Front. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.-K.; Wang, H. Waning immune responses against SARS-CoV-2 among vaccinees in Hong Kong. eBioMedicine 2022, 77, 103904. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Salimi-Jeda, A.; Abbassi, S.; Mousavizadeh, A.; Esghaie, M.; Bokharaei-Salim, F.; Jeddi, F.; Shafaati, M.; Abdoli, A. SARS-CoV-2: Current trends in emerging variants, pathogenesis, immune responses, potential therapeutic, and vaccine development strategies. Int. Immunopharmacol. 2021, 101, 108232. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shayestehpour, M.; Mirzaei, H. The impact of spike mutated variants of SARS-CoV2 [Alpha, Beta, Gamma, Delta, and Lambda] on the efficacy of subunit recombinant vaccines. Braz. J. Infect. Dis. 2021, 25, 101606. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 2020, 182, 1284–1294.e1289. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nie, J.; Wu, J.; Zhang, L.; Ding, R.; Wang, H.; Zhang, Y.; Li, T.; Liu, S.; Zhang, M. SARS-CoV-2 501Y. V2 variants lack higher infectivity but do have immune escape. Cell 2021, 184, 2362–2371.e2369. [Google Scholar] [CrossRef]
- Bian, L.; Gao, F.; Zhang, J.; He, Q.; Mao, Q.; Xu, M.; Liang, Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 365–373. [Google Scholar] [CrossRef]
- Chen, L.-L.; Lu, L.; Choi, C.Y.-K.; Cai, J.-P.; Tsoi, H.-W.; Chu, A.W.-H.; Ip, J.D.; Chan, W.-M.; Zhang, R.R.; Zhang, X. Impact of SARS-CoV-2 variant-associated RBD mutations on the susceptibility to serum antibodies elicited by COVID-19 infection or vaccination. Clin. Infect. Dis. 2021, ciab656. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Lan, T.X.; He, X.M.; Liu, J.; Chen, L.; Baptista-Hon, D.T.; Zhang, K.; Wei, X.W. SARS-CoV-2 Omicron variant: Immune escape and vaccine development. MedComm 2022, 3, e126. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.-W.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B. 1.1. 7 and B. 1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.; Moodley, D.; Hanley, S. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Kimura, I.; Shirakawa, K.; Takaori-Kondo, A.; Nakada, T.-A.; Kaneda, A.; Nakagawa, S.; Sato, K. Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. N. Engl. J. Med. 2021, 385, 2379–2399. [Google Scholar] [CrossRef]
- Kalathiya, U.; Padariya, M.; Fahraeus, R.; Chakraborti, S.; Hupp, T.R. Multivalent display of SARS-CoV-2 spike (RBD domain) of COVID-19 to nanomaterial, protein ferritin nanocages. Biomolecules 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, J.; Wei, G.-W. Mechanisms of SARS-CoV-2 evolution revealing vaccine-resistant mutations in Europe and America. J. Phys. Chem. Lett. 2021, 12, 11850–11857. [Google Scholar] [CrossRef]
- Meier, S.; Güthe, S.; Kiefhaber, T.; Grzesiek, S. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable β-hairpin: Atomic details of trimer dissociation and local β-hairpin stability from residual dipolar couplings. J. Mol. Biol. 2004, 344, 1051–1069. [Google Scholar] [CrossRef]
- Wang, X.H.R.; Cao, T.; Sun, C.; Luo, D.; Qiu, H.; Wu, M.; Huang, X.; Yu, C.; Li, J.; Kong, D.; et al. Development of a Thermostable, Bivalent Recombinant Trimer Spike Protein Vaccine against SARS-CoV-2 Alpha and Beta Variants. 2022; submitted. [Google Scholar]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Su, D.; Li, X.; He, C.; Huang, X.; Chen, M.; Wang, Q.; Qin, W.; Liang, Y.; Xu, R.; Wu, J. Broad neutralization against SARS-CoV-2 variants induced by a modified B. 1.351 protein-based COVID-19 vaccine candidate. bioRxiv, 2021; Preprint. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); Statpearls Publishing: Treasure Island, FL, USA, 2022; Chapter Etiology and Treatment/Management. [Google Scholar]
- Moyo-Gwete, T.; Madzivhandila, M.; Makhado, Z.; Ayres, F.; Mhlanga, D.; Oosthuysen, B.; Lambson, B.E.; Kgagudi, P.; Tegally, H.; Iranzadeh, A. Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y. V2 (B. 1.351). N. Engl. J. Med. 2021, 384, 2161–2163. [Google Scholar] [CrossRef]
- Reed, C.C.; Schultheis, K.; Andrade, V.M.; Kalia, R.; Tur, J.; Schouest, B.; Elwood, D.; Walters, J.N.; Maricic, I.; Doan, A. Design, immunogenicity and efficacy of a Pan-SARS-CoV-2 synthetic DNA vaccine. bioRxiv, 2021; Preprint. [Google Scholar] [CrossRef]
- Sarkar, R.; Lo, M.; Saha, R.; Dutta, S.; Chawla-Sarkar, M. S glycoprotein diversity of the Omicron variant. medRxiv, 2021; Preprint. [Google Scholar] [CrossRef]
- Bolles, M.; Deming, D.; Long, K.; Agnihothram, S.; Whitmore, A.; Ferris, M.; Funkhouser, W.; Gralinski, L.; Totura, A.; Heise, M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011, 85, 12201–12215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Wang, S.-H.; Tang, Y.; Sheng, W.; Zuo, C.-J.; Wu, D.-W.; Fang, H.; Du, Q.; Li, N. Landscape and progress of global COVID-19 vaccine development. Hum. Vaccines Immunother. 2021, 17, 3276–3280. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e2379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M. Neutralizing activity of BNT162b2-elicited serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- Supasa, P.; Zhou, D.; Dejnirattisai, W.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.; Nutalai, R.; Tuekprakhon, A. Reduced neutralization of SARS-CoV-2 B. 1.1. 7 variant by convalescent and vaccine sera. Cell 2021, 184, 2201–2211.e2207. [Google Scholar] [CrossRef]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.; Tuekprakhon, A.; Nutalai, R. Evidence of escape of SARS-CoV-2 variant B. 1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361.e2346. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.-V.; Pinsky, B.A.; Suthar, M.S.; Lai, L.; Davis-Gardner, M.E.; Floyd, K.; Flowers, M.W.; Wrammert, J.; Hussaini, L.; Ciric, C.R. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B. 1.617 variants. N. Engl. J. Med. 2021, 385, 664–666. [Google Scholar] [CrossRef]
- Fact Sheet for Health Care Providers Emergency Use Authorization (Eua) of Bamlanivimab AND Etesevimab (fda.gov). Available online: https://www.fda.gov/media/145802/download (accessed on 30 September 2021).
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y. Reduced neutralization of SARS-CoV-2 B. 1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e4213. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Choi, A.; Koch, M.; Elbashir, S.; Ma, L.; Lee, D.; Woods, A.; Henry, C.; Palandjian, C.; Hill, A. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. Vaccine 2021, 39, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.G.; Chen, W.-H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Martinez, E.J.; Chang, W.C.; Peterson, C.E.; Morrison, E.B. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 2021, 37, 110143. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.G.; King, H.A.; Elakhal-Naouar, I.; Ahmed, A.; Peachman, K.K.; Cincotta, C.M.; Subra, C.; Chen, R.E.; Thomas, P.V.; Chen, W.-H. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci. Transl. Med. 2021, eabi5735. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, X.; Lin, J.; Ai, J.; Yang, J.; Hu, Z.; Fu, Y.-X.; Peng, H. Broad neutralization against SARS-CoV-2 variants induced by a next-generation protein vaccine V-01. Cell Discov. 2021, 7, 1–3. [Google Scholar] [CrossRef]
- Martinez, D.R.; Schäfer, A.; Leist, S.R.; De la Cruz, G.; West, A.; Atochina-Vasserman, E.N.; Lindesmith, L.C.; Pardi, N.; Parks, R.; Barr, M. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science 2021, 373, 991–998. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yang, J.; He, X.; Hong, W.; Lei, H.; Chen, Z.; Shen, G.; Yang, L.; Li, J.; Wang, Z. A bivalent recombinant vaccine targeting the S1 protein induces neutralizing antibodies against both SARS-CoV-2 variants and wild-type of the virus. MedComm 2021, 2, 430–441. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, X.; Chen, R.; Li, Y.; Wu, B.; Li, R.; Zou, F.; Ma, X.; Wang, X.; Chen, Q. A bivalent nanoparticle vaccine exhibits potent cross-protection against the variants of SARS-CoV-2. Cell Rep. 2022, 38, 110256. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-M.; Liu, M.-C.; Chen, Y.-H.; Lee, W.-S.; Hwang, S.-J.; Cheng, S.-H.; Ko, W.-C.; Hwang, K.-P.; Wang, N.-C.; Lee, Y.-L. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- Tsai, T.F. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infect. Chemother. 2013, 45, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Valdez, A.; Valdez-Zapata, G.; Patel, S.S.; Castelli, F.V.; Garcia, M.G.; Jansen, W.T.; Arora, A.K.; Heijnen, E. MF59-adjuvanted influenza vaccine (FLUAD®) elicits higher immune responses than a non-adjuvanted influenza vaccine (Fluzone®): A randomized, multicenter, Phase III pediatric trial in Mexico. Hum. Vaccines Immunother. 2018, 14, 386–395. [Google Scholar] [CrossRef]
- Kavian, N.; Hachim, A.; Li, A.P.; Cohen, C.A.; Chin, A.W.; Poon, L.L.; Fang, V.J.; Leung, N.H.; Cowling, B.J.; Valkenburg, S.A. Assessment of enhanced influenza vaccination finds that FluAd conveys an advantage in mice and older adults. Clin. Transl. Immunol. 2020, 9, e1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, B.W.; Kim, C.O.; Izu, A.; Arora, A.K.; Heijnen, E. Phase 4, post-marketing safety surveillance of the MF59-adjuvanted influenza vaccines Fluad® and Vantaflu® in South Korean subjects aged ≥65 years. Infect. Chemother. 2018, 50, 301–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Sun, C.; Ma, J.; Yu, C.; Kong, D.; Chen, M.; Liu, X.; Zhao, D.; Gao, S.; Kou, S.; et al. A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants. Vaccines 2022, 10, 702. https://doi.org/10.3390/vaccines10050702
Wang R, Sun C, Ma J, Yu C, Kong D, Chen M, Liu X, Zhao D, Gao S, Kou S, et al. A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants. Vaccines. 2022; 10(5):702. https://doi.org/10.3390/vaccines10050702
Chicago/Turabian StyleWang, Rui, Chunyun Sun, Juan Ma, Chulin Yu, Desheng Kong, Meng Chen, Xuejie Liu, Dandan Zhao, Shuman Gao, Shuyuan Kou, and et al. 2022. "A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants" Vaccines 10, no. 5: 702. https://doi.org/10.3390/vaccines10050702
APA StyleWang, R., Sun, C., Ma, J., Yu, C., Kong, D., Chen, M., Liu, X., Zhao, D., Gao, S., Kou, S., Sun, L., Ge, Z., Zhao, J., Li, K., Zhang, T., Zhang, Y., Luo, C., Li, X., Wang, Y., & Xie, L. (2022). A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants. Vaccines, 10(5), 702. https://doi.org/10.3390/vaccines10050702





