Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID-19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population
Abstract
:1. Introduction
2. Materials and Methods
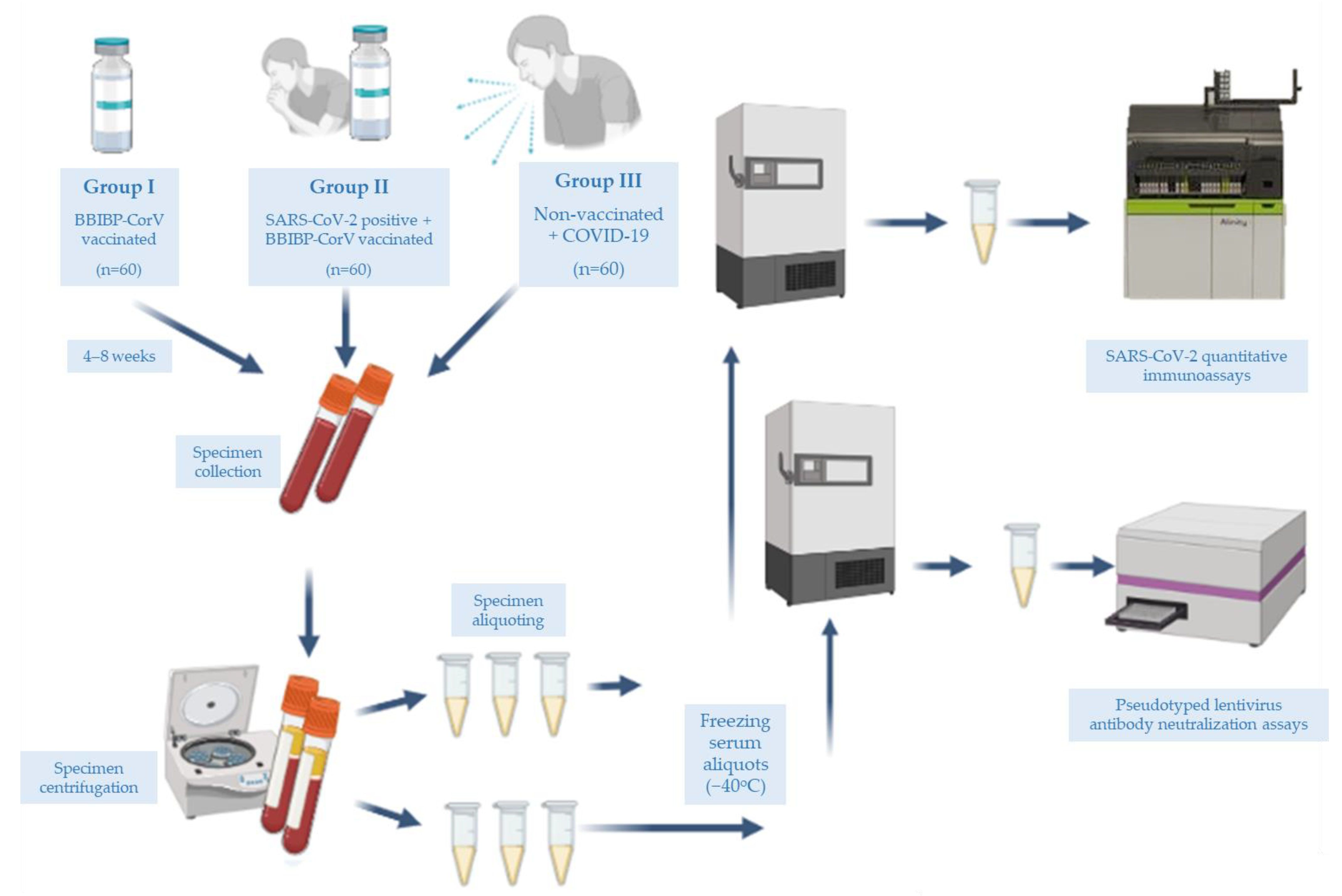
2.1. Participants and Specimen Collection
2.2. Immunoassay
2.3. Cell Culture
2.4. Plasmids
2.5. Lentivirus Preparation
2.6. Lentivirus Titration
2.7. Pseudotyped Lentivirus Antibody Neutralization Assay
2.8. Data Analysis
2.9. Validation of the Pseudotyped Lentivirus Antibody Neutralization Assay
3. Results and Discussion
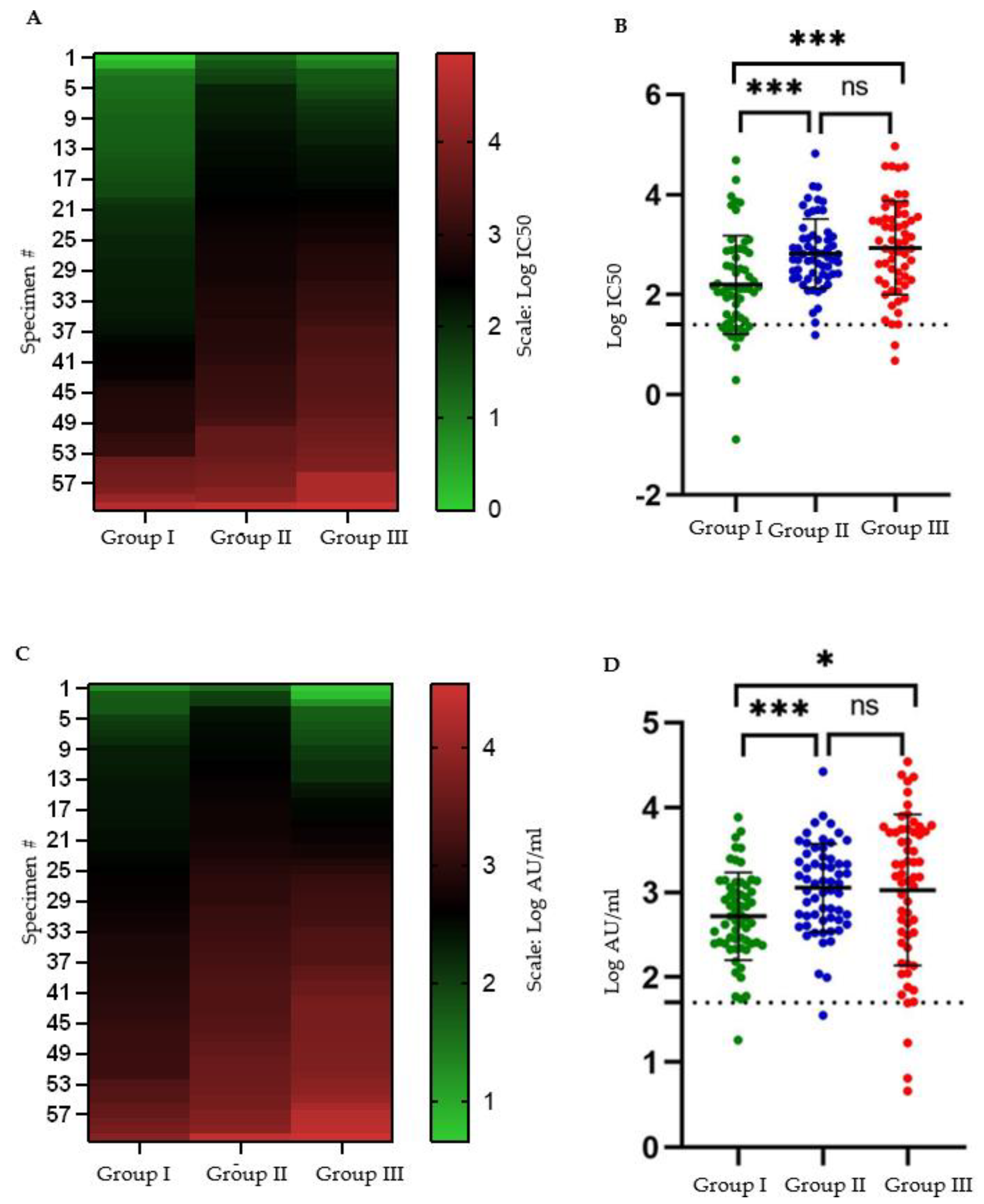
3.1. Seroconversion Rate following Two-Dose BBIBP-CorV Protocol Is Lower than That following Moderate to Severe COVID-19
3.2. Mean Antibody Titers following Two-Dose BBIBP-CorV Protocol Are Lower than That following Moderate to Severe COVID-19
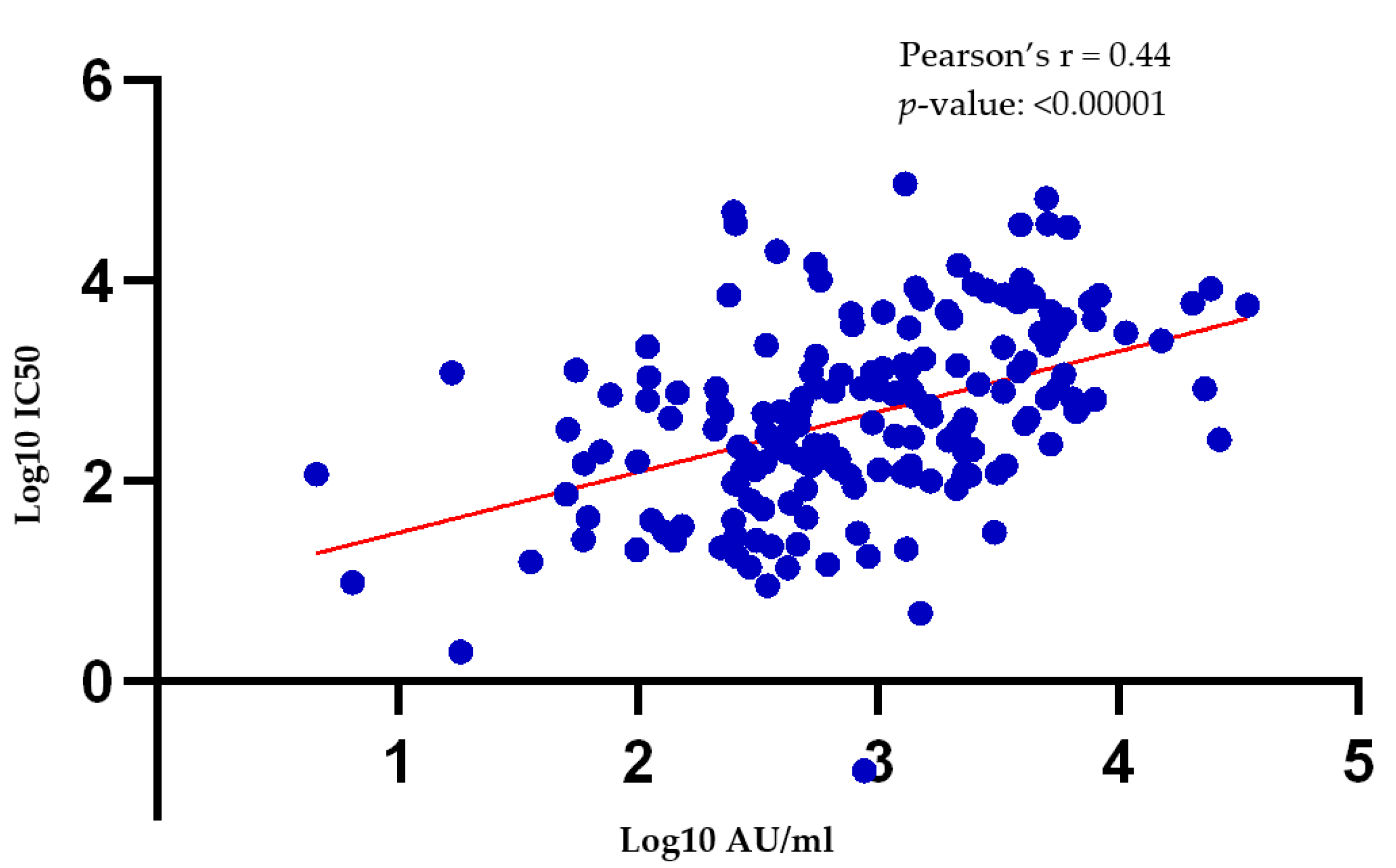
3.3. There Is a Moderate Correlation between Antibody Titers Obtained via the Pseudotyped Lentivirus Antibody Neutralization Assay and the SARS-CoV-2 Quant II Immunoassay (Abbott)
3.4. No Significant Differences in the Mean Antibody Titers of the Study Participants Were Observed Based on Their Sex or Age Bracket
3.5. The Interval between Sample Collection and the Second Vaccine Dose (Group I and Group II)/Onset of Symptoms (Group III) Had No Correlation with Antibody Titers
3.6. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Vaccine Tracker COVID. Available online: https://covid19.trackvaccines.org/ (accessed on 18 March 2022).
- Kudlay, D.; Svistunov, A. COVID-19 Vaccines: An Overview of Different Platforms. Bioengineering 2022, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Shi, J.; Wang, Z.; Zhou, S.; Jin, Y.; Zheng, Z.J. Disparities in COVID-19 Vaccination among Low-, Middle-, and High-Income Countries: The Mediating Role of Vaccination Policy. Vaccines 2021, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Achieving 70% COVID-19 Immunization Coverage by Mid-2022. Available online: https://www.who.int/news/item/23-12-2021-achieving-70-covid-19-immunization-coverage-by-mid-2022 (accessed on 19 March 2022).
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953, Epub 2021 May 10; Erratum in: Nat Hum Behav. 2021 June 17. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. Npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.Y.L.; Pang, A.S.R.; Chow, V.T.; Wang, D.Y.l. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Nixon, D.F.; Moore, J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021, 7, eabe8065. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Condor Capcha, J.M.; Lambert, G.; Dykxhoorn, D.M.; Salerno, A.G.; Hare, J.M.; Whitt, M.A.; Pahwa, S.; Jayaweera, D.T.; Shehadeh, L.A. Generation of SARS-CoV-2 Spike Pseudotyped Virus for Viral Entry and Neutralization Assays: A 1-Week Protocol. Front. Cardiovasc. Med. 2021, 7, 618651. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef]
- Lamikanra, A.; Nguyen, D.; Simmonds, P.; Williams, S.; Bentley, E.M.; Rowe, C.; Otter, A.D.; Brooks, T.; Gilmour, K.; Mai, A.; et al. Comparability of six different immunoassays measuring SARS-CoV-2 antibodies with neutralizing antibody levels in convalescent plasma: From utility to prediction. Transfusion 2021, 61, 2837–2843. [Google Scholar] [CrossRef]
- Gul, A. Pakistan Starts COVID-19 Inoculation Drive VOA. 2021. Available online: https://www.voanews.com/a/covid-19-pandemic_pakistan-starts-covid-19-inoculation-drive/6201529.html (accessed on 18 March 2022).
- COVID. Available online: https://covid19.trackvaccines.org/country/pakistan/ (accessed on 18 March 2022).
- Pakistan Approves China’s Sinopharm COVID-19 Vaccine for Emergency Use Xinhua. Available online: http://www.xinhuanet.com/english/2021-01/19/c_139679907.htm (accessed on 18 March 2022).
- Ians Pakistan to Purchase Covid-19 Vaccine from China’s Sinopharm. Business Standard. 2020. Available online: https://www.business-standard.com/article/current-affairs/pakistan-to-purchase-covid-19-vaccine-from-china-s-sinopharm-120123100641_1.html (accessed on 18 March 2022).
- Farooq, U. Pakistan Health Workers Hesitate over Sinopharm Vaccine, Poll Says Reuters. 2021. Available online: https://www.reuters.com/article/us-health-coronavirus-pakistan-idUSKBN2AX1QR (accessed on 18 March 2022).
- Who Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. World Health Organization. Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 18 March 2022).
- Ferenci, T.; Sarkadi, B. RBD-specific antibody responses after two doses of BBIBP-CorV (Sinopharm, Beijing CNBG) vaccine. BMC Infect. Dis. 2022, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Clinical Spectrum. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 18 March 2022).
- Crawford, K. Pseudotyping Lentiviral Particles with SARS-COV-2 Spike Protein for Neutralization Assays. Protocols.io. 2021. Available online: https://www.protocols.io/view/pseudotyping-lentiviral-particles-with-sars-cov-2-j8nlke2r5l5r/v2 (accessed on 18 March 2022).
- Crawford, K.H.D.; Eguia, R.; Dingens, A.S.; Loes, A.N.; Malone, K.D.; Wolf, C.R.; Chu, H.Y.; Tortorici, M.A.; Veesler, D.; Murphy, M.; et al. Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses 2020, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Tolan, N.V.; Sherman, A.C.; Zhou, G.; Nabel, K.G.; Desjardins, M.; Melanson, S.; Kanjilal, S.; Moheed, S.; Kupelian, J.; Kaufman, R.M.; et al. The Effect of Vaccine Type and SARS-CoV-2 Lineage on Commercial SARS-CoV-2 Serologic and Pseudotype Neutralization Assays in mRNA Vaccine Recipients. Microbiol. Spectr. 2022, 21, e0021122. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, S.; Liang, D.; Hu, W.; Ke, C.; He., J.; Yuan., R.; Huang., Y.; Li., Y.; Liu., D.; et al. Differential Antibody Response to Inactivated COVID-19 Vaccines in Healthy Subjects. Front. Cell. Infect. Microbiol. 2021, 11, 791660. [Google Scholar] [CrossRef]
- Li, M.; Jiang, R.; Wang, E.; Xiong, D.; Ou, T.; Zhang, X.; Dou, X. Performance evaluation of an automatic chemiluminescence immune platform for SARS-CoV-2 neutralizing antibody after vaccination in real world. BMC Infect. Dis. 2022, 22, 157. [Google Scholar] [CrossRef]
- Petrović, V.; Vuković, V.; Patić, A.; Marković, M.; Ristić, M. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS-CoV-2 infection-A comparative study from Novi Sad, Serbia. PLoS ONE 2022, 17, e0263468. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Pushpakumara, P.D.; Kamaladasa, A.; Guruge, D.; Wijesinghe, A.; Gunasekera, B.; Tanussiya, S.; Kruppu, H.; Ranasinghe, T.; et al. Persistence of antibody and T cell responses to the Sinopharm/BBIBP-CorV vaccine in Sri Lankan individuals. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, F.; Cui, L.; Zhao, Y.; Zhang, H.; Fu, S.; Zhang, J. Immunological Analysis of People in Northeast China after SARS-CoV-2 Inactivated Vaccine Injection. Vaccines 2021, 9, 1028. [Google Scholar] [CrossRef]
- Elgendy, M.O.; El-Gendy, A.O.; Mahmoud, S.; Mohammed, T.Y.; Abdelrahim, M.E.A.; Sayed, A.M. Side Effects and Efficacy of COVID-19 Vaccines among the Egyptian Population. Vaccines 2022, 10, 109. [Google Scholar] [CrossRef]
- Elgendy, M.O.; El-Gendy, A.O.; Alzarea, A.I.; Mahmoud, S.; Alqahtani, S.S.; Fahmy, A.M.; El-Seedi, H.R.; Sayed, A.M.; Alatawi, A.D.; Abdelrahim, M.E.A.; et al. SARS-CoV-2 Post Vaccinated Adverse Effects and Efficacy in the Egyptian Population. Vaccines 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.E.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef]
- Lijeskić, O.; Klun, I.; Stamenov Djaković, M.; Gligorić, N.; Štajner, T.; Srbljanović, J.; Djurković-Djaković, O. Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report. Vaccines 2021, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Badano, M.N.; Sabbione, F.; Keitelman, I.; Pereson, M.; Aloisi, N.; Colado, A.; Ramos, M.V.; Ortiz Wilczyñski, J.M.; Pozner, R.G.; Castillo, L.; et al. Humoral response to the BBIBP-CorV vaccine over time in healthcare workers with or without exposure to SARS-CoV-2. Mol. Immunol. 2022, 143, 94–99. [Google Scholar] [CrossRef] [PubMed]
- El-Ghitany, E.M.; Hashish, M.H.; Farag, S.; Omran, E.A.; Farghaly, A.G. Azzam NFAE. Determinants of the Development of SARS-CoV-2 Anti-Spike Immune-Response after Vaccination among Healthcare Workers in Egypt. Vaccines 2022, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.L.; Shi, D.W.; Li, Y.; Hong, W.; Lai, D.Y.; Xue, J.B.; Jiang, H.W.; Zhang, H.N.; Qi, H.; Meng, Q.F.; et al. Systematic profiling of SARS-CoV-2-specific IgG responses elicited by an inactivated virus vaccine identifies peptides and proteins for predicting vaccination efficacy. Cell Discov. 2021, 7, 67. [Google Scholar] [CrossRef]
- Hu, C.; Li, D.; Liu, Z.; Ren, L.; Su, J.; Zhu, M.; Feng, Y.; Wang, Z.; Liu, Q.; Zhu, B.; et al. Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations. Pathogens 2022, 11, 171. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Miédougé, M.; Abravanel, F.; Da Silva, I.; Porcheron, M.; Fillaux, J.; Diméglio, C.; Izopet, J. Evaluation of Three Quantitative Anti-SARS-CoV-2 Antibody Immunoassays. Microbiol. Spectr. 2021, 9, e0137621. [Google Scholar] [CrossRef]
- Feng, X.; Yin, J.; Zhang, J.; Hu, Y.; Ouyang, Y.; Qiao, S.; Zhao, H.; Zhang, T.; Li, X.; Zhang, L.; et al. Longitudinal Profiling of Antibody Response in Patients With COVID-19 in a Tertiary Care Hospital in Beijing, China. Front. Immunol. 2021, 12, 614436. [Google Scholar] [CrossRef]
- Glück, V.; Grobecker, S.; Tydykov, L.; Salzberger, B.; Glück, T.; Weidlich, T.; Bertok, M.; Gottwald, C.; Wenzel, J.J.; Gessner, A. SARS-CoV-2-directed antibodies persist for more than six months in a cohort with mild to moderate COVID-19. Infection 2021, 49, 739–746. [Google Scholar] [CrossRef]
- Longueira, Y.; Polo, M.L. InViV working group; Biobanco de Enfermedades Infecciosas Colección COVID19 working group, Turk, G.; Laufer, N. Dynamics of SARS-CoV-2-specific antibodies among COVID19 biobank donors in Argentina. Heliyon 2021, 7, e08140. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Qiao, N.; Wang, X.; Ding, L.; Zhu, X.; Liang, Y.; Han, Z.; Liu, F.; Zhang, X.; et al. Early assessment of the safety and immunogenicity of a third dose (booster) of COVID-19 immunization in Chinese adults. Front. Med. 2022, 16, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Huang, H.; Zheng, P.; Xue, M.; Ma, J.; Zhan, Z.; Gan, H.; Zeng, Y.; Lin, R.; Li, S.; et al. Humoral immune response of Sinopharm/BBIBP COVID-19 vaccination before and after the booster immunization. Allergy 2022, in press. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Song, J.; Wu, J.; Zhu, Y.; Li, M.; Cui, Y.; Chen, Y.; Yang, L.; Liu, J.; et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg. Microbes Infect. 2022, 11, 477–481. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; Van Blargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Zhou, W.; He, P.; Li, J.; Liu, H.; Shi, M.; Yu, J.; Wei, H. Steep Decline in Binding Capability of SARS-CoV-2 Omicron Variant (B.1.1.529) RBD to the Antibodies in Early COVID-19 Convalescent Sera and Inactivated Vaccine Sera. Viruses 2022, 14, 335. [Google Scholar] [CrossRef]
- Huang, B.; Dai, L.; Wang, H.; Hu, Z.; Yang, X.; Tan, W.; Gao, G.F. Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y.V2. Lancet Microbe 2021, 2, e285. [Google Scholar] [CrossRef]
- Hadj Hassine, I. Covid-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2021, e2313. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Y.; Tang, L.; Wang, F.; Ye, Y.; Ma, C.; Zheng, H.; Yu, W.; Cao, L.; Song, Y.; et al. Effectiveness of Inactivated COVID-19 Vaccines Against Symptomatic, Pneumonia, and Severe Disease Caused by the Delta Variant: Real World Study and Evidence-China, 2021. China CDC Wkly. 2022, 4, 57–65. [Google Scholar] [CrossRef] [PubMed]


| Sex | Age Brackets (Years) | ||||||
|---|---|---|---|---|---|---|---|
| F | M | 18–29 | 30–39 | 40–49 | 50–59 | 60 & > | |
| Group I | 26 | 34 | 12 | 23 | 16 | 8 | 1 |
| Group II | 29 | 31 | 24 | 20 | 15 | 1 | 0 |
| Group III | 19 | 41 | 15 | 10 | 7 | 10 | 18 |
| Total | 74 | 106 | 51 | 53 | 38 | 19 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aijaz, J.; Hussain, S.; Naseer, F.; Kanani, F.; Anis, S.; Sarfaraz, S.; Saeed, S.; Farooq, H.; Jamal, S. Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID-19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population. Vaccines 2022, 10, 692. https://doi.org/10.3390/vaccines10050692
Aijaz J, Hussain S, Naseer F, Kanani F, Anis S, Sarfaraz S, Saeed S, Farooq H, Jamal S. Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID-19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population. Vaccines. 2022; 10(5):692. https://doi.org/10.3390/vaccines10050692
Chicago/Turabian StyleAijaz, Javeria, Shakir Hussain, Fouzia Naseer, Fatima Kanani, Sabiha Anis, Samreen Sarfaraz, Saima Saeed, Hina Farooq, and Saba Jamal. 2022. "Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID-19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population" Vaccines 10, no. 5: 692. https://doi.org/10.3390/vaccines10050692
APA StyleAijaz, J., Hussain, S., Naseer, F., Kanani, F., Anis, S., Sarfaraz, S., Saeed, S., Farooq, H., & Jamal, S. (2022). Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID-19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population. Vaccines, 10(5), 692. https://doi.org/10.3390/vaccines10050692






