Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants
Abstract
:1. Introduction
1.1. Cells, Virus and Animals
1.2. Western Blotting
1.3. Immunofluorescence Assay
1.4. Virus Inactivation
1.5. Preparation of Inactivated Vaccines
1.6. Vaccine Immunization of Pig
1.7. Samples Collection
1.8. Neutralization Assay
1.9. Quantitative Real-Time PCR
1.10. Histopathological Examination
1.11. Statistical Analysis
2. Results
2.1. Characteristics of SVV LNSY01-2017 on BHK-21 Cells
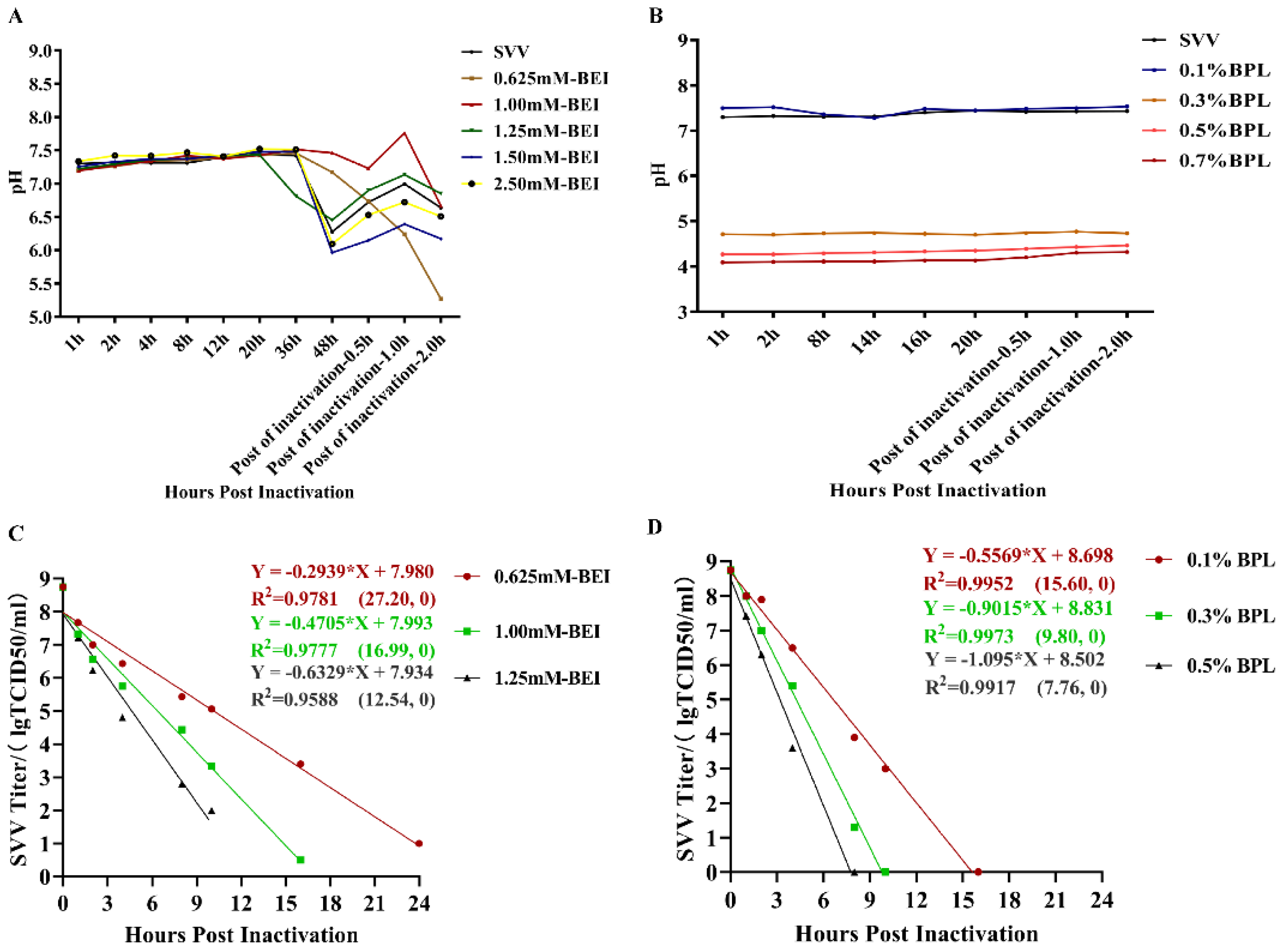
2.2. Inactivation of SVV LNSY01-2017 with BEI and BPL
2.3. Detection Antigen (VP1) of BEI/BPL Inactivated Samples
2.4. Immune Response of the SVV LNSYO1-2017 Inactivated Vaccines in Pigs
2.5. Vesicular Lesion and Clinical Scores Observed in Pigs Post-Challenge Infection
2.6. Histopathological Analysis of Post-Challenge Infection
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, L.M.; Knowles, N.J.; Reddy, P.S.; Xu, L.; Hay, C.; Hallenbeck, P.L. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J. Gen. Virol. 2008, 89 Pt 5, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Maggioli, M.F.; Lawson, S.; de Lima, M.; Joshi, L.R.; Faccin, T.C.; Bauermann, F.V.; Diel, D.G. Adaptive Immune Responses following Senecavirus A Infection in Pigs. J. Virol. 2018, 92, e01717-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Zhu, H.; Li, S.; Shi, M.; Zhou, E.; Wang, X.; Jiang, P.; Bai, J. Identification of linear B cell epitopes on VP1 and VP2 proteins of Senecavirus A (SVA) using monoclonal antibodies. Veter. Microbiol. 2020, 247, 108753. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, F.A.; Linhares, D.C.L.; de Barcellos, D.E.S.N.; Lam, H.C.; Collins, J.; Marthaler, D. Identification and Complete Genome of Seneca Valley Virus in Vesicular Fluid and Sera of Pigs Affected with Idiopathic Vesicular Disease, Brazil. Transbound. Emerg. Dis. 2015, 62, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Oliveira, T.E.; Alcântara, B.K.; Headley, S.A.; Alfieri, A.F.; Yang, M.; Alfieri, A.A. Clinical Manifestations of Senecavirus A Infection in Neonatal Pigs, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1238–1241. [Google Scholar] [CrossRef]
- Leme, R.A.; Oliveira, T.E.S.; Alfieri, A.F.; Headley, S.A.; Alfieri, A.A. Pathological, Immunohistochemical and Molecular Findings Associated with Senecavirus A-Induced Lesions in Neonatal Piglets. J. Comp. Pathol. 2016, 155, 145–155. [Google Scholar] [CrossRef]
- Leme, R.A.; Alfieri, A.F.; Alfieri, A.A. Update on Senecavirus Infection in Pigs. Viruses 2017, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Joshi, L.R.; Fernandes, M.H.V.; Clement, T.; Lawson, S.; Pillatzki, A.; Resende, T.P.; Vannucci, F.A.; Kutish, G.F.; Nelson, E.A.; Diel, D.G. Pathogenesis of Senecavirus A infection in finishing pigs. J. Gen. Virol. 2016, 97, 3267–3279. [Google Scholar] [CrossRef]
- Joshi, L.R.; Mohr, K.A.; Clement, T.; Hain, K.S.; Myers, B.; Yaros, J.; Nelson, E.A.; Christopher-Hennings, J.; Gava, D.; Schaefer, R.; et al. Detection of the Emerging Picornavirus Senecavirus A in Pigs, Mice, and Houseflies. J. Clin. Microbiol. 2016, 54, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Saeng-Chuto, K.; Rodtian, P.; Temeeyasen, G.; Wegner, M.; Nilubol, D. The first detection of Senecavirus A in pigs in Thailand, 2016. Transbound. Emerg. Dis. 2018, 65, 285–288. [Google Scholar] [CrossRef]
- Sun, D.; Vannucci, F.; Knutson, T.P.; Corzo, C.; Marthaler, D. Emergence and whole-genome sequence of Senecavirus A in Colombia. Transbound. Emerg. Dis. 2017, 64, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, J.; Ba, L.; Wang, F.; Gao, D.; Zhang, J.; Pan, C.; Qi, P. Identification and genomic characterization of the emerging Senecavirus A in southeast China, 2017. Transbound. Emerg. Dis. 2018, 65, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, F.; Chen, P.; Liu, H.; Cao, W.; Zhang, K.; Liu, X.; Zheng, H. Emergence of novel Seneca Valley virus strains in China, 2017. Transbound. Emerg. Dis. 2017, 64, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.M.T.; Akkutay-Yoldar, Z.; Stone, S.R.; Tousignant, S.J.; Vannucci, F.A.; Murtaugh, M.P. An indirect enzyme-linked immunosorbent assay for the identification of antibodies to Senecavirus A in swine. BMC Veter. Res. 2016, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Van Bruggen, R.; Xu, W. Generation and diagnostic application of monoclonal antibodies against Seneca Valley virus. J. Veter. Diagn. Investig. 2012, 24, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Fowler, V.L.; Ransburgh, R.H.; Poulsen, E.G.; Wadsworth, J.; King, D.; Mioulet, V.; Knowles, N.J.; Williamson, S.; Liu, X.; Anderson, G.A.; et al. Development of a novel real-time RT-PCR assay to detect Seneca Valley virus-1 associated with emerging cases of vesicular disease in pigs. J. Virol. Methods 2017, 239, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Goolia, M.; Vannucci, F.; Yang, M.; Patnayak, D.; Babiuk, S.; Nfon, C.K. Validation of a competitive ELISA and a virus neutralization test for the detection and confirmation of antibodies to Senecavirus A in swine sera. J. Veter. Diagn. Investig. 2017, 29, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Bracht, A.J.; O’Hearn, E.S.; Fabian, A.W.; Barrette, R.W.; Sayed, A. Real-Time Reverse Transcription PCR Assay for Detection of Senecavirus A in Swine Vesicular Diagnostic Specimens. PLoS ONE 2016, 11, e0146211. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, Z.; Cao, W.; Liu, H.; Zhang, K.; Tian, H.; Liu, X.; Zheng, H. Immunogenicity and protective efficacy of an inactivated cell culture-derived Seneca Valley virus vaccine in pigs. Vaccine 2018, 36, 841–846. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, P.; Hao, G.; Liu, W.; Chen, H.; Qian, P.; Li, X. Comparison of the Pathogenicity of Two Different Branches of Senecavirus a Strain in China. Pathogens 2020, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhang, R.; Liu, T.; Sun, Z.; Hu, M.; Sun, Y.; Cheng, L.; Guo, Y.; Fu, S.; Hu, J.; et al. Seneca Valley virus attachment and uncoating mediated by its receptor anthrax toxin receptor 1. Proc. Natl. Acad. Sci. USA 2018, 115, 13087–13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746. [Google Scholar] [CrossRef]
- Vellozzi, C.; Burwen, D.R.; Dobardzic, A.; Ball, R.; Walton, K.; Haber, P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine 2009, 27, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Diaz-San Segundo, F.; Medina, G.N.; Stenfeldt, C.; Arzt, J.; de Los Santos, T. Foot-and-mouth disease vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef]
- Doel, T.R. FMD vaccines. Virus Res. 2003, 91, 81–99. [Google Scholar] [CrossRef]
- Bahnemann, H.G. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch. Virol. 1975, 47, 47–56. [Google Scholar] [CrossRef]
- Chowdhury, P.; Topno, R.; Khan, S.A.; Mahanta, J. Comparison of β-Propiolactone and Formalin Inactivation on Antigenicity and Immune Response of West Nile Virus. Adv. Virol. 2015, 2015, 616898. [Google Scholar] [CrossRef] [Green Version]
- Budowsky, E.I.; Smirnov Yu, A.; Shenderovich, S.F. Principles of selective inactivation of viral genome. VIII. The influence of beta-propiolactone on immunogenic and protective activities of influenza virus. Vaccine 1993, 11, 343–348. [Google Scholar] [CrossRef]
- Budowsky, E.I.; Friedman, E.A.; Zheleznova, N.V.; Noskov, F.S. Principles of selective inactivation of viral genome. VI. Inactivation of the infectivity of the influenza virus by the action of beta-propiolactone. Vaccine 1991, 9, 398–402. [Google Scholar] [CrossRef]
- Lei, S.; Gao, X.; Sun, Y.; Yu, X.; Zhao, L. Gas chromatography-mass spectrometry method for determination of β-propiolactone in human inactivated rabies vaccine and its hydrolysis analysis. J. Pharm. Anal. 2018, 8, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fernandes, M.H.V.; De Lima, M.; Joshi, L.R.; Lawson, S.; Diel, D.G. A Novel Live Attenuated Vaccine Candidate Protects Against Heterologous Senecavirus A Challenge. Front. Immunol. 2019, 10, 2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Zhu, Z.; Liu, H.; Cao, W.; Zhang, W.; Wei, T.; Zheng, M.; Zhang, K.; Tian, H.; Zeng, Q.; et al. Evaluation of Antibody Response in Sows after Vaccination with Senecavirus A Vaccine and the Effect of Maternal Antibody Transfer on Antibody Dynamics in Offspring. Vaccines 2021, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xiao, P.; Kong, D.; Liu, X.; Meng, L.; An, T.; Cai, X.; Wang, H.; Yu, L. Engineering His-Tagged Senecavirus A for One-Step Purification of Viral Antigens. Vaccines 2022, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liao, Y.; Sun, Y.; Ruan, Y.; Liu, C.; Zhang, M.; Li, F.; Li, X.; Fan, S.; et al. Preliminary Evaluation of Protective Efficacy of Inactivated Senecavirus A on Pigs. Life 2021, 11, 157. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Li, X.; Zhang, H.; Hao, G.; Shang, X.; Wang, H.; Chen, H.; Qian, P. Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants. Vaccines 2022, 10, 631. https://doi.org/10.3390/vaccines10040631
Liu W, Li X, Zhang H, Hao G, Shang X, Wang H, Chen H, Qian P. Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants. Vaccines. 2022; 10(4):631. https://doi.org/10.3390/vaccines10040631
Chicago/Turabian StyleLiu, Wenqiang, Xiangmin Li, Huawei Zhang, Genxi Hao, Xianfei Shang, Huilan Wang, Huanchun Chen, and Ping Qian. 2022. "Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants" Vaccines 10, no. 4: 631. https://doi.org/10.3390/vaccines10040631
APA StyleLiu, W., Li, X., Zhang, H., Hao, G., Shang, X., Wang, H., Chen, H., & Qian, P. (2022). Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants. Vaccines, 10(4), 631. https://doi.org/10.3390/vaccines10040631




