COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family?
Abstract
:1. Introduction
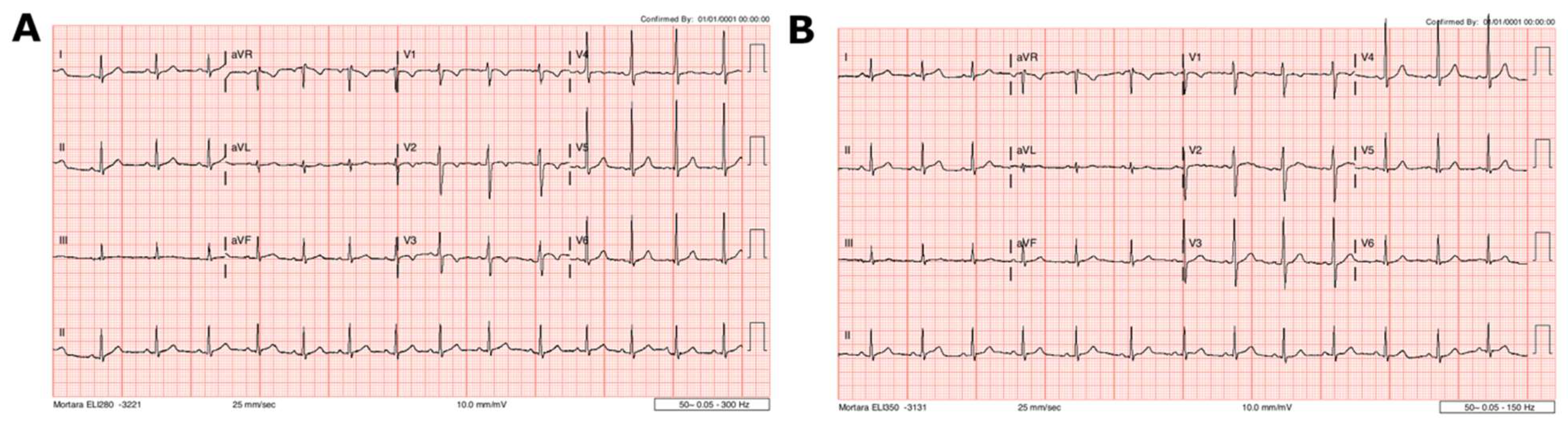
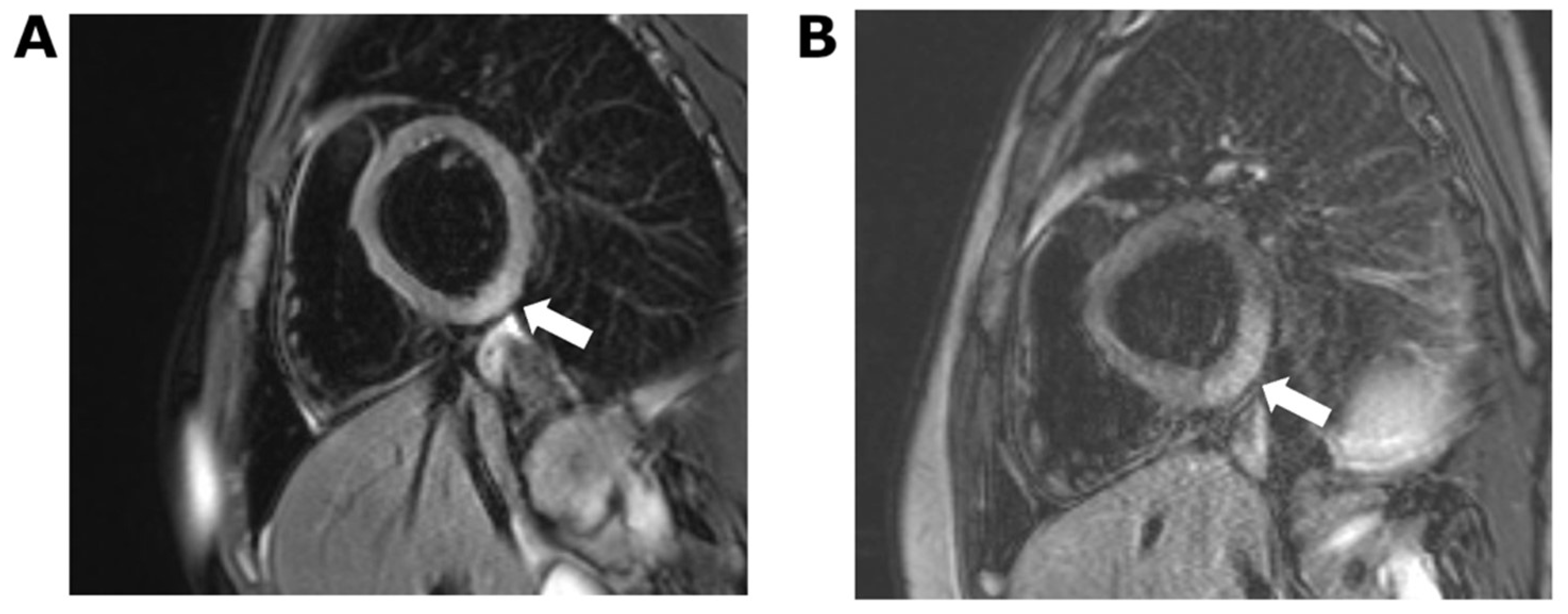
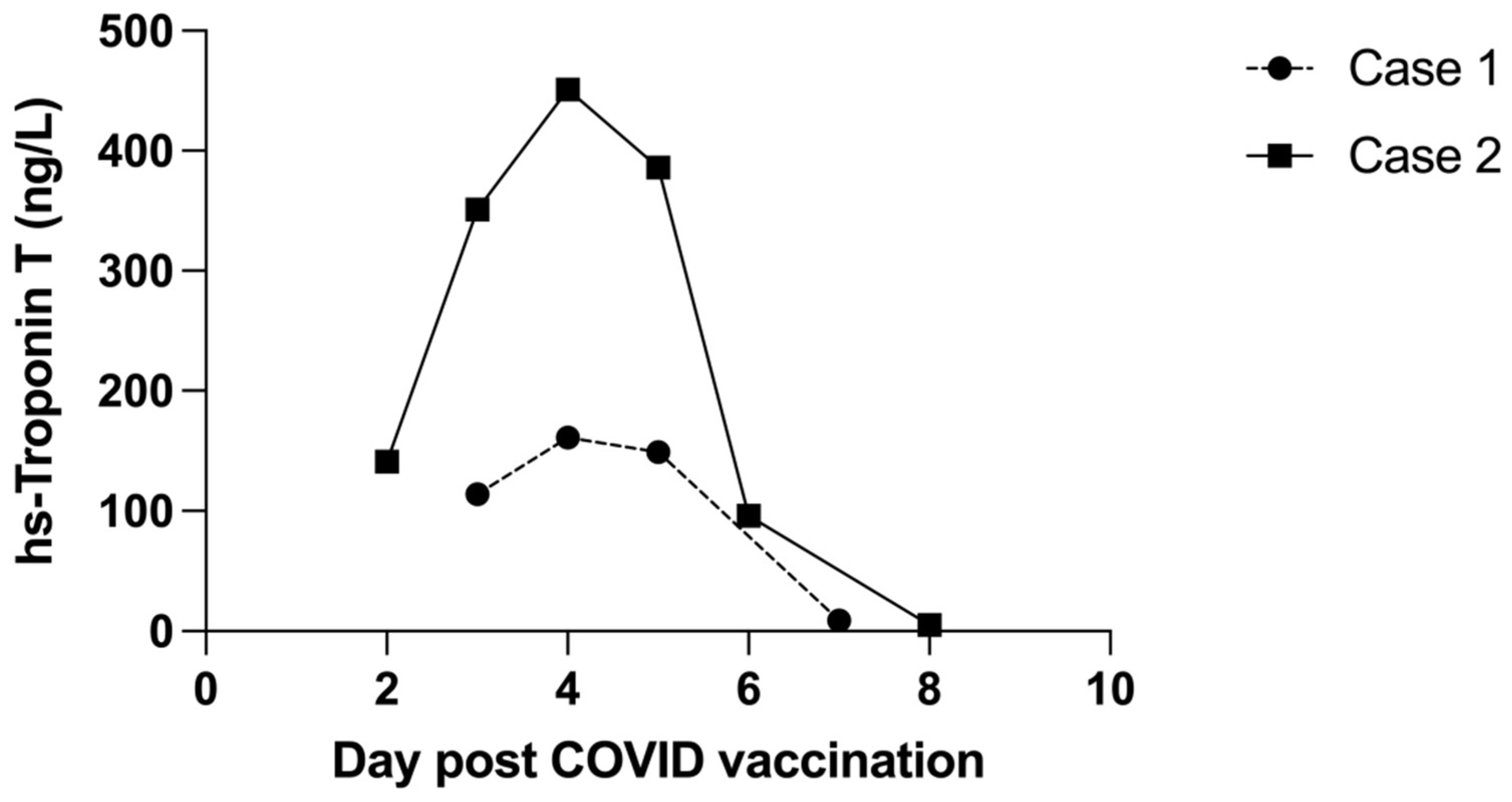
2. Case 1
3. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Shimabukuro, T.T.; DeStefano, F. Myocarditis Occurring after Immunization with mRNA-Based COVID-19 Vaccines. JAMA Cardiol. 2021, 6, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Simone, A.; Herald, J.; Chen, A.; Gulati, N.; Shen, A.Y.; Lewin, B.; Lee, M.S. Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern. Med. 2021, 181, 1668–1670. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Cramer, J.P.; Dekker, C.L.; Dudley, M.Z.; Graham, B.S.; Gurwith, M.; Law, B.; Perlman, S.; Polack, F.P.; Spergel, J.M.; et al. Vaccine-associated enhanced disease: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3053–3066. [Google Scholar] [CrossRef]
- Truong, D.T.; Dionne, A.; Muniz, J.C.; McHugh, K.E.; Portman, M.A.; Lambert, L.M.; Thacker, D.; Elias, M.D.; Li, J.S.; Toro-Salazar, O.H.; et al. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults. Circulation 2021, 145, 345–356. [Google Scholar] [CrossRef]
- Levin, D.; Shimon, G.; Fadlon-Derai, M.; Gershovitz, L.; Shovali, A.; Sebbag, A.; Bader, S.; Fink, N.; Gordon, B. Myocarditis following COVID-19 vaccination—A case series. Vaccine 2021, 39, 6195–6200. [Google Scholar] [CrossRef]
- COVID-19: Vaccine Data. Available online: https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-coronavirus/covid-19-data-and-statistics/covid-19-vaccine-data (accessed on 1 March 2022).
- Chin, S.E.; Bhavsar, S.M.; Corson, A.; Ghersin, Z.J.; Kim, H.S. Cardiac Complications Associated with COVID-19, MIS-C, and mRNA COVID-19 Vaccination. Pediatr. Cardiol. 2022, 43, 483–488. [Google Scholar] [CrossRef]
- Won, T.; Gilotra, N.A.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Lovell, J.; Milstone, A.M.; Steenbergen, C.; Čiháková, D. Increased Interleukin 18-Dependent Immune Responses Are Associated with Myopericarditis after COVID-19 mRNA Vaccination. Front. Immunol. 2022, 13, 851620. [Google Scholar] [CrossRef]
- Saeed, S.; Käsk, L.; Rajani, R.; Larsen, T.H. Incidence, clinical presentation and management of myocarditis following mRNA-based Covid-19 vaccines: A brief report. Cardiology 2022. [Google Scholar] [CrossRef]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; Del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef]
- Australian Technical Advisory Group on Immunisation (ATAGI); the Cardiac Society of Australia and New Zealand (CSANZ); the Royal Australian College of General Practitioners (RACGP); the Australian College of Remote and Rural Medicine (ACRRM); the Australasian College of Emergency (ACEM). Guidance on Myocarditis and Pericarditis after mRNA COVID-19 Vaccines; Australian Government: Canberra, Australia, 2021.
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Aggarwal, S.; Kheiwa, A.; Shah, N. Myopericarditis in children: Elevated troponin I level does not predict outcome. Pediatr. Cardiol. 2012, 33, 1040–1045. [Google Scholar] [CrossRef]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef]
- Viskin, D.; Topilsky, Y.; Aviram, G.; Mann, T.; Sadon, S.; Hadad, Y.; Flint, N.; Shmilovich, H.; Banai, S.; Havakuk, O. Myocarditis Associated With COVID-19 Vaccination: Echocardiography, Cardiac Tomography, and Magnetic Resonance Imaging Findings. Circ. Cardiovasc. Imaging 2021, 14, e013236. [Google Scholar] [CrossRef]
- Jain, S.S.; Steele, J.M.; Fonseca, B.; Huang, S.; Shah, S.; Maskatia, S.A.; Buddhe, S.; Misra, N.; Ramachandran, P.; Gaur, L.; et al. COVID-19 Vaccination-Associated Myocarditis in Adolescents. Pediatrics 2021, 148, e2021053427. [Google Scholar] [CrossRef]
- Chelala, L.; Jeudy, J.; Hossain, R.; Rosenthal, G.; Pietris, N.; White, C. Cardiac MRI Findings of Myocarditis After COVID-19 mRNA Vaccination in Adolescents. AJR Am. J. Roentgenol. 2021, 218, 651–657. [Google Scholar] [CrossRef]
- Manfredi, R.; Bianco, F.; Bucciarelli, V.; Ciliberti, G.; Guerra, F.; Schicchi, N.; Tavio, M.; Berton, E.; Surace, F.C.; Colaneri, M.; et al. Clinical Profiles and CMR Findings of Young Adults and Pediatrics with Acute Myocarditis Following mRNA COVID-19 Vaccination: A Case Series. Vaccines 2022, 10, 169. [Google Scholar] [CrossRef]
- Campuzano, O.; Fernández-Falgueras, A.; Sarquella-Brugada, G.; Sanchez, O.; Cesar, S.; Mademont, I.; Allegue, C.; Mates, J.; Pérez-Serra, A.; Coll, M.; et al. A Genetically Vulnerable Myocardium May Predispose to Myocarditis. J. Am. Coll. Cardiol. 2015, 66, 2913–2914. [Google Scholar] [CrossRef] [Green Version]
- Baggio, C.; Gagno, G.; Porcari, A.; Paldino, A.; Artico, J.; Castrichini, M.; Dal Ferro, M.; Bussani, R.; Merlo, M. Myocarditis: Which Role for Genetics? Curr. Cardiol. Rep. 2021, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Artico, J.; Merlo, M.; Delcaro, G.; Cannatà, A.; Gentile, P.; De Angelis, G.; Paldino, A.; Bussani, R.; Ferro, M.D.; Sinagra, G. Lymphocytic Myocarditis: A Genetically Predisposed Disease? J. Am. Coll. Cardiol. 2020, 75, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Ligons, D.L.; Rose, N.R. Genetic complexity of autoimmune myocarditis. Autoimmun. Rev. 2008, 7, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | |
|---|---|---|
| Patient anthropometrics | ||
| Age (years) | 14 | 12 |
| Weight (kg) | 55.3 | 54.5 |
| Height (cm) | 164 | 162 |
| BMI (kg/m2) | 20.4 | 20.8 |
| Vital parameters at presentation | ||
| Heart rate (bpm) | 88 | 85 |
| Blood pressure (mmHg) | 117/79 | 103/58 |
| SpO2 (%) | 98 | 99 |
| Laboratory parameters at presentation | ||
| White blood cell count (E + 9/L) | 5.39 | 5.57 |
| Lymphocyte count (E + 9/L) | 2.68 | 2.23 |
| C-reactive protein (mg/L) | 4.6 | 1.5 |
| Haemoglobin (g/L) | 143 | 138 |
| COVID-19 history | ||
| No previous recorded COVID-19 infection Parents received COVID-19 Pfizer-BioNTech vaccination without side effects | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosmann, J.; Gentles, T.; Occleshaw, C.; Mitchelson, B. COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family? Vaccines 2022, 10, 611. https://doi.org/10.3390/vaccines10040611
Moosmann J, Gentles T, Occleshaw C, Mitchelson B. COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family? Vaccines. 2022; 10(4):611. https://doi.org/10.3390/vaccines10040611
Chicago/Turabian StyleMoosmann, Julia, Thomas Gentles, Christopher Occleshaw, and Bryan Mitchelson. 2022. "COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family?" Vaccines 10, no. 4: 611. https://doi.org/10.3390/vaccines10040611
APA StyleMoosmann, J., Gentles, T., Occleshaw, C., & Mitchelson, B. (2022). COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family? Vaccines, 10(4), 611. https://doi.org/10.3390/vaccines10040611






