An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cell culture as a substrate for the production of influenza vaccines: Memorandum from a WHO meeting. Bull. World Health Organ. 1995, 73, 431–435. [Google Scholar]
- Joint Committee on Vaccination and Immunisation. Minute of the Meeting Held on 27 October 2020. Teleconference. Available online: https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation#minutes (accessed on 14 June 2021).
- Seqirus UK Ltd. Prescribing Information: Cell-Based Quadrivalent (Surface Antigen, Inactivated). 2021. Available online: https://labeling.seqirus.com/API/UK/QIVc/EN/QIVc-Prescribing-Information.pdf (accessed on 15 January 2022).
- Sanofi Pasteur. Prescribing Information: Supemtek Solution for Infection in Pre-Filled Syringe. Quadrivalent Influenza Vaccine (Recombinant, Prepared in Cell Culture). 2021. Available online: https://www.sanofipasteur.co.uk/-/media/ems/conditions/vaccines/brands/oneportal-ie/pdf/supemtek.pdf (accessed on 15 January 2022).
- Joint Committee on Vaccination and Immunisation (JCVI) Scientific Secretariat. JCVI Advice on Influenza Vaccines for the 2019/2020 Influenza Season. 2018. Available online: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (accessed on 5 December 2018).
- Joint Committee on Vaccination and Immunisation (JCVI) Scientific Secretariat. JCVI Advice on Influenza Vaccines for the 2020/21 Influenza season. 2019. Available online: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (accessed on 2 October 2020).
- Joint Committee on Vaccination and Immunisation (JCVI) Scientific Secretariat. JCVI Advice on Influenza Vaccines for the 2021/22 Influenza Season. 2020. Available online: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (accessed on 17 December 2020).
- Joint Committee on Vaccination and Immunisation (JCVI) Scientific Secretariat. JCVI Advice on Influenza Vaccines for the 2022/2023 Influenza Season. 2021. Available online: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (accessed on 22 September 2021).
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2019 to 2020; Public Health England: London, UK, 2020.
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; Team, P.S.C.S. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- Dawood, F.S.; Naleway, A.L.; Flannery, B.; Levine, M.Z.; Murthy, K.; Sambhara, S.; Gangappa, S.; Edwards, L.; Ball, S.; Beacham, L.; et al. Comparison of the Immunogenicity of Cell Culture-Based and Recombinant Quadrivalent Influenza Vaccines to Conventional Egg-Based Quadrivalent Influenza Vaccines among Healthcare Personnel Aged 18–64 Years: A Randomized Open-Label Trial. Clin. Infect. Dis. 2021, 73, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Murchu, E.O.; Comber, L.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Ryan, M.; Teljeur, C.; Carnahan, A.; Perez, J.J.; et al. Systematic review of the efficacy, effectiveness and safety of recombinant haemagglutinin seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals >/=18 years of age. Rev. Med. Virol. 2022, e2331. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. The National Flu Immunisation Programme 2020 to 2021—Update (Letter: 5 August 2020). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907149/Letter_annualflu_2020_to_2021_update.pdf (accessed on 15 December 2020).
- National Health Service. Vaccines Reimbursed as Part of the NHS Seasonal Influenza Immunisation Programme 2021/22 (Letter: 1 April 2021). Available online: https://www.england.nhs.uk/wp-content/uploads/2021/04/BW485-achievements-and-developments-during-202021-flu-season.pdf (accessed on 16 April 2021).
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Ashraf, M.; Drummond, M.F.; Weinstein, M.C. The cost-effectiveness of expanding vaccination with a cell-based influenza vaccine to low risk adults aged 50 to 64 years in the United Kingdom. Vaccines 2021, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Drummond, M.; Weinstein, M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum Vaccines Immunother. 2021, 17, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9]. 2013. Available online: https://www.nice.org.uk/process/pmg9/chapter/foreword (accessed on 15 February 2019).
- Cromer, D.; van Hoek, A.J.; Jit, M.; Edmunds, W.J.; Fleming, D.; Miller, E. The burden of influenza in England by age and clinical risk group: A statistical analysis to inform vaccine policy. J. Infect. 2014, 68, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguelin, M.; Jit, M.; Miller, E.; Edmunds, W.J. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine 2012, 30, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Tilston, N.L.; Eames, K.T.; Paolotti, D.; Ealden, T.; Edmunds, W.J. Internet-based surveillance of Influenza-like-illness in the UK during the 2009 H1N1 influenza pandemic. BMC Public Health 2010, 10, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitman, R.J.; Nagy, L.D.; Sculpher, M.J. Cost-effectiveness of childhood influenza vaccination in England and Wales: Results from a dynamic transmission model. Vaccine 2013, 31, 927–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguelin, M.; Camacho, A.; Flasche, S.; Edmunds, W.J. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: A cost-effectiveness study. BMC Med. 2015, 13, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hoek, A.J.; Underwood, A.; Jit, M.; Miller, E.; Edmunds, W.J. The impact of pandemic influenza H1N1 on health-related quality of life: A prospective population-based study. PLoS ONE 2011, 6, e17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melegaro, A.; Edmunds, W.J. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 2004, 22, 4203–4214. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. National Life Tables: United Kingdom, 2015–2017. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed on 23 April 2019).
- Meier, G.; Gregg, M.; Poulsen Nautrup, B. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: An updated analysis in the U.K. J. Med. Econ. 2015, 18, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Thorrington, D.; van Leeuwen, E.; Ramsay, M.; Pebody, R.; Baguelin, M. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine 2019, 37, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Thorrington, D.; van Leeuwen, E.; Ramsay, M.; Pebody, R.; Baguelin, M. Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England. BMC Med. 2017, 15, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguelin, M.; Flasche, S.; Camacho, A.; Demiris, N.; Miller, E.; Edmunds, W.J. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013, 10, e1001527. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunisation (JCVI). Code of Practice. 2013. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/224864/JCVI_Code_of_Practice_revision_2013_-_final.pdf (accessed on 3 December 2021).
- Health Protection Agency. Surveillance of Influenza and Other Respiratory Viruses in the UK, 2010/11; Health Protection Agency: London, UK, 2011.
- Health Protection Agency. Surveillance of Influenza and Other Respiratory Pathogens in the UK. October 2011–April 2012; Health Protection Agency: London, UK, 2012.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses, Including Novel Respiratory Viruses, in the United Kingdom: Winter 2012/13; Public Health England: London, UK, 2013.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the United Kingdom: Winter 2013/14; Public Health England: London, UK, 2014.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the United Kingdom: Winter 2014 to 2015; Public Health England: London, UK, 2015.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the United Kingdom: Winter 2015 to 2016; Public Health England: London, UK, 2016.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2016 to 2017; Public Health England: London, UK, 2017.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2017 to 2018; Public Health England: London, UK, 2018.
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2018 to 2019; Public Health England: London, UK, 2019.
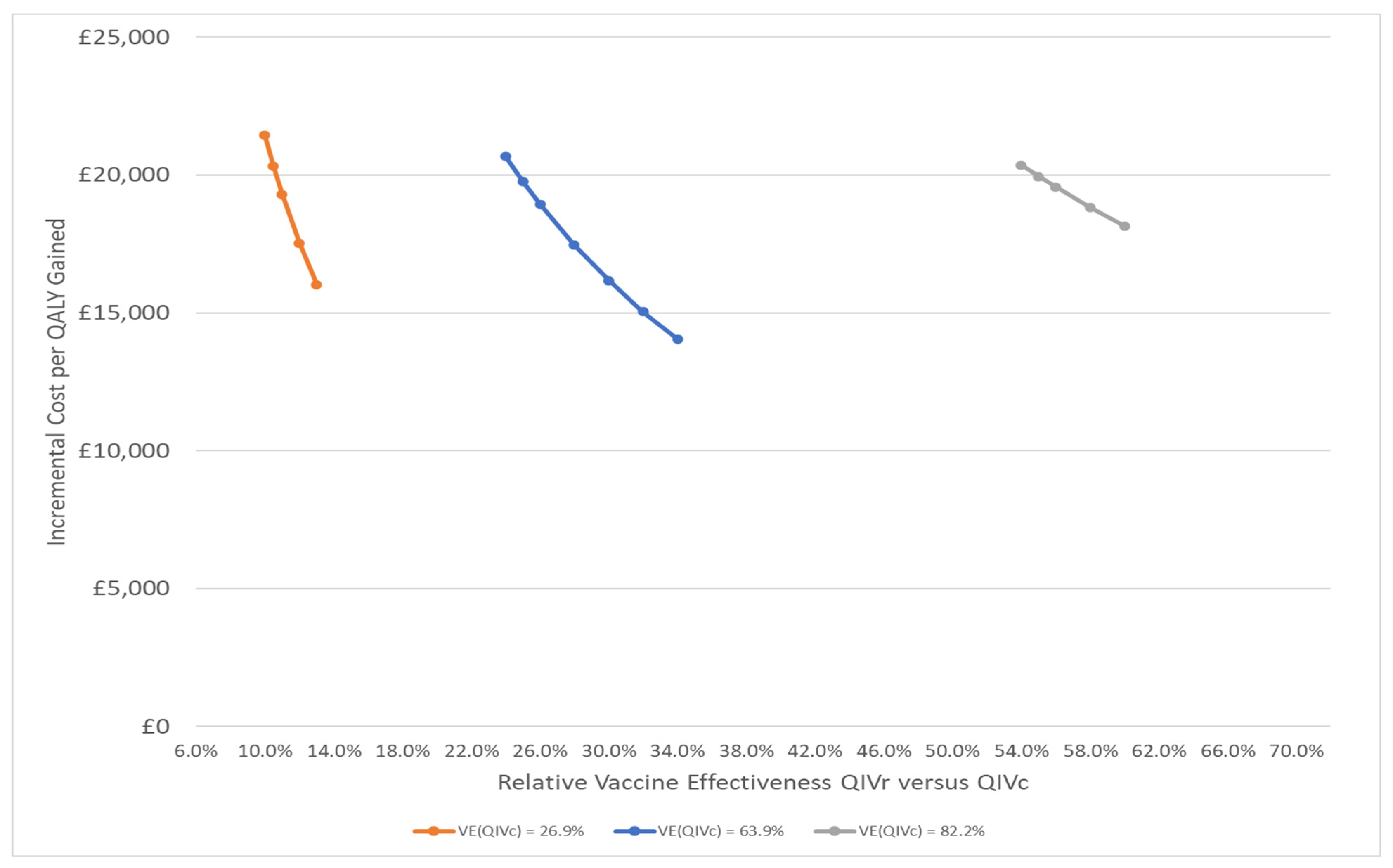
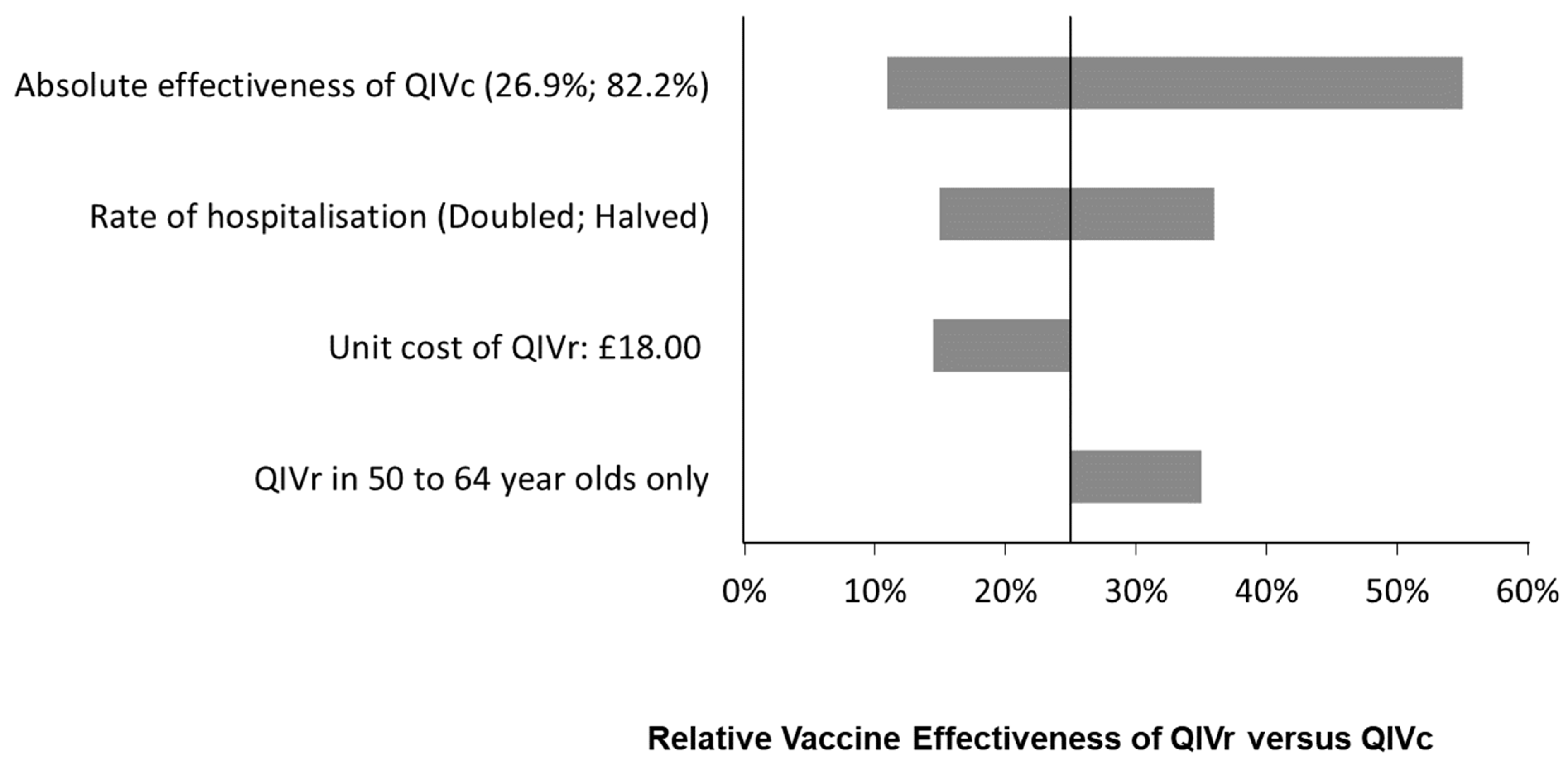
| Age Group | Percent of Population at High Risk of Complication if Infected | Vaccine Coverage (%) | |
|---|---|---|---|
| Low Risk | High Risk | ||
| 6–23 months | 4.90% | 0.10% | 3.10% |
| 2–6 years 1 | 7.30% | 28.10% | 48.60% |
| 7–17 years 1 | 9.60% | 27.60% | 48.60% |
| 18–49 years | 9.10% | 0.00% | 48.60% |
| 50–59 years | 18.30% | 34.0% | 52.00% |
| 60–64 years | 18.30% | 34.0% | 52.00% |
| 65–74 years | 45.00% | 81.00% | 81.00% |
| 75 years and above | 45.00% | 81.00% | 81.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maschio, M.; Kohli, M.A.; Ashraf, M.; Drummond, M.F.; Weinstein, M.C.; Mould-Quevedo, J.F. An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom. Vaccines 2022, 10, 599. https://doi.org/10.3390/vaccines10040599
Maschio M, Kohli MA, Ashraf M, Drummond MF, Weinstein MC, Mould-Quevedo JF. An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom. Vaccines. 2022; 10(4):599. https://doi.org/10.3390/vaccines10040599
Chicago/Turabian StyleMaschio, Michael, Michele A. Kohli, Mansoor Ashraf, Michael F. Drummond, Milton C. Weinstein, and Joaquin F. Mould-Quevedo. 2022. "An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom" Vaccines 10, no. 4: 599. https://doi.org/10.3390/vaccines10040599
APA StyleMaschio, M., Kohli, M. A., Ashraf, M., Drummond, M. F., Weinstein, M. C., & Mould-Quevedo, J. F. (2022). An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom. Vaccines, 10(4), 599. https://doi.org/10.3390/vaccines10040599






