1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) belongs to the family
Coronaviridae, genus
Betacoronavirus, and subgenus
Sarbecovirus. Since it is different from both zoonotic coronavirus MERS-CoV and SARS-CoV introduced to humans earlier in the past two decades, it has also been called a novel coronavirus [
1]. The first case report of a patient infected with this novel coronavirus came from Wuhan, China [
2], and at that time, the World Health Organization (WHO) released the official name of the disease caused by this virus, as coronavirus disease 19 (COVID-19) [
3]. On 11 March 2020, the WHO declared COVID-19 as a pandemic [
4]. Social distancing and travel restrictions began to come into force along with advice on effective handwashing techniques. The WHO initially recommended the use of masks only by those taking care of infected individuals, but later advised their use even for healthy individuals in community settings [
5].
Clinical testing of broad-spectrum antivirals and other drugs for the treatment of COVID-19 infection and trials of the first COVID-19 human mRNA vaccine began simultaneously in March 2020. The safety and immunogenicity of two candidates’ vaccines, BNT162b1 encoding a secreted trimerized SARS-CoV-2 receptor–binding domain and BNT162b2 encoding a membrane-anchored SARS-CoV-2 full-length spike, stabilized in the prefusion conformation, were tested in a phase I study [
6]. Both vaccines elicited similar dose-dependent SARS-CoV-2–neutralizing geometric mean titers, but BNT162b2 was associated with a lower incidence and severity of systemic reactions. This supported the selection of BNT162b2 for advancement to a phase II/III trial [
7], which showed 95% efficacy in preventing COVID-19 and similar safety to that of other viral vaccines at a median of 2 months. Through 6 months of follow-up, BNT162b2 remained highly efficacious and displayed a favorable safety profile [
8].
COVID-19 vaccines induce innate and adaptive immune responses by different mechanisms involving the T cellular response and the B cellular response, which leads to the production of antibodies directed against SARS-CoV-2 antigens [
9]. Antibodies against both spike and nucleocapsid antigens are produced to neutralize structural proteins of the virus. Spike (S) is a transmembrane glycoprotein comprised of S1 and S2 regions. S1 region mediates recognition and binding on host cells by interacting with the angiotensin-converting enzyme human 2 (ACE2) receptor, S2 region facilitates fusion and entry of a virus. The majority of S1 is comprised of the receptor-binding domain (RBD), which binds directly to ACE2 and is highly immunogenic [
10,
11]. The BNT162b1 vaccine induced robust immune responses [
12,
13], but data were published at short median follow-up. An assessment of the dynamics of antibody response and CD4
+ or CD8
+ T cell responses up to 6 months after vaccination with two doses of the BNT162b2 mRNA vaccine was published recently [
14].
The objective of this study was to prove the efficacy of vaccination, monitor the adverse events of two BNT162b2 doses, determine IgG plasma levels at 3 weeks followed by weeks 7 and 26, and explore immune cell response at 6 months after double vaccination in a specific population of staff at the National Comprehensive Cancer Center (NCCC) in Slovakia.
3. Discussion
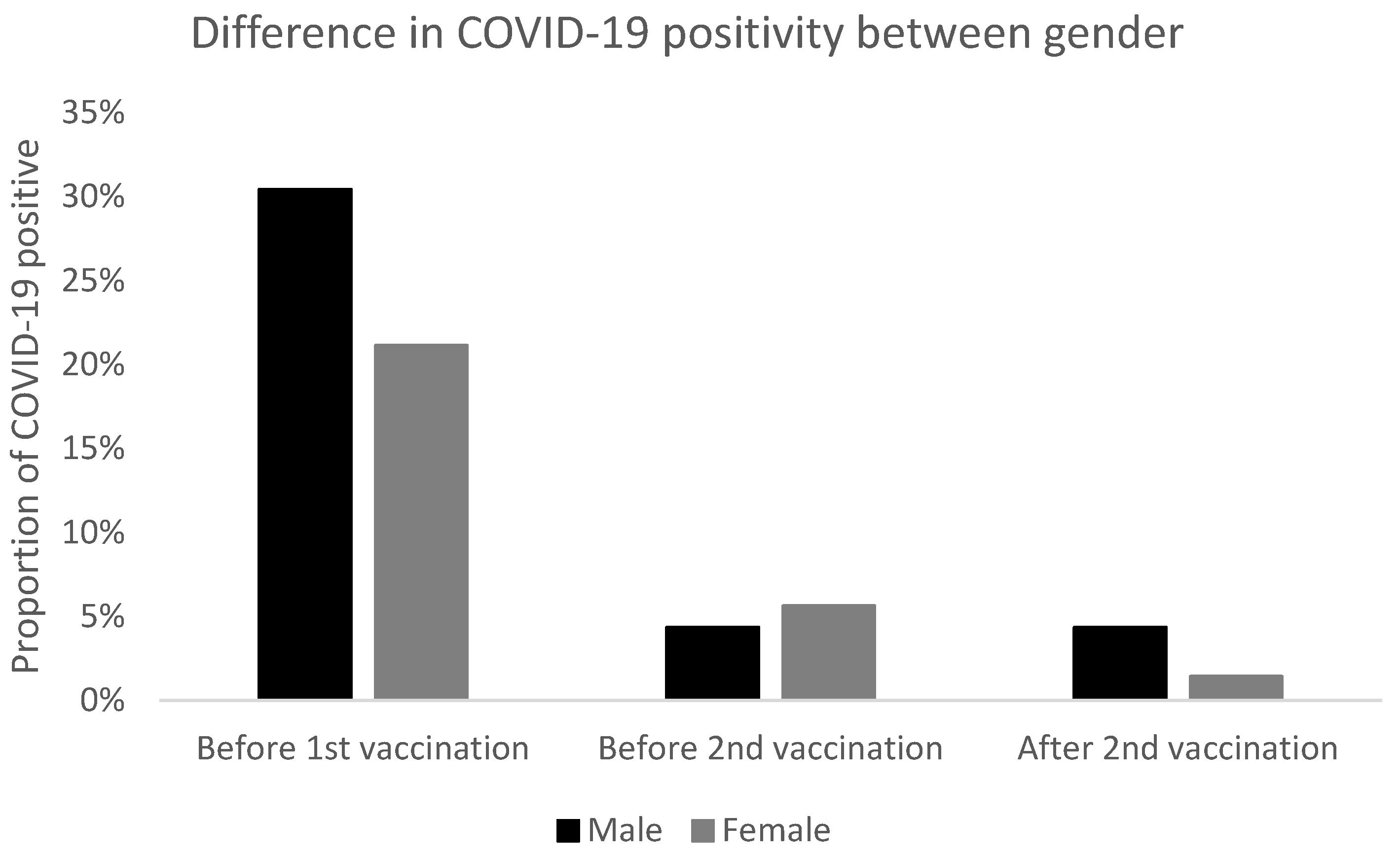
In this study, we confirmed high vaccine efficacy as defined by the number of COVID-19 positive subjects at or after day 7 following the second dose of BNT162b2 (97.87%). Moreover, we recorded significantly more patients with COVID-19 before the first dose of vaccine compared to the time after double vaccination with BNT162b2. Regarding gender, there were no significant differences, however, COVID-19 was significantly more often diagnosed before vaccination in subjects with morbid obesity than normal weight and after the second vaccination in overweight vs. normal weight.
In a population of 94 NCCC staff, two cases of COVID-19 positivity were recorded despite double vaccination. The first case was a 29-year-old female nurse who tested positive for COVID-19 Delta variant 191 days after the second vaccine dose application by RT-PCR (CT E gene of 15.9). Seven days before positive testing, the measured IgG antibodies were 14.95 index. Despite a high viral load (tonsil swab) and low IgG antibody plasma level, the course of the disease was mild, including a fever up to 38.1 °C, chills, sore throat, headache, and clogged nasal cavities. All symptoms and signs disappeared within 2 days after symptomatic treatment (paracetamol, naproxen, and calcium syrup). After infection, the level of virus-neutralizing antibodies was not investigated, but cell-mediated immunity was determined within this study 73 days after RT-PCR positive testing and concluded as borderline.
The second case was a 49-year-old male physician who tested positive for COVID-19 Delta variant 256 days after the second vaccine dose administration by RT-PCR (CT E gene of 30.0). The IgG antibodies measured 173 days after the second vaccine dose were 7.36 index. Cell-mediated immunity determined 244 days after double vaccination was concluded as negative. In this person, the course of the disease was mild to moderate, including a fever up to 39.0 °C, generalized arthralgia and myalgia, headache, and dry cough. Symptoms and signs disappeared over 4 days. On day 3 after positive testing, this patient was given a monoclonal antibody bamlanivimab 700 mg in a single, 1-h, intravenous infusion. The virus neutralizing antibodies were not investigated after infection.
The high effectiveness of BNT162b2 is consistent with 6 months of follow-up among the participants aged 16 years and older in a large placebo-controlled phase III trial [
8], in which a vaccine efficacy of 86% to 100% was seen across countries and populations of differing age, gender, ethnic group, and risk factors (such as high BMI) for COVID-19 among individuals without evidence of previous infection with the novel coronavirus. Vaccine efficacy against severe disease was 96.7%. Healthcare workers are a population worthy of special consideration due to recognized high exposure and their potential role in the transmission of SARS-CoV-2. In the SIREN study [
16], the effectiveness of the BNT162b2 vaccine was 85% seven days after two doses in a cohort of healthcare workers undergoing regular asymptomatic testing. In both previously mentioned trials, the same definition of BNT162b2 vaccine effectiveness was used as in our study.
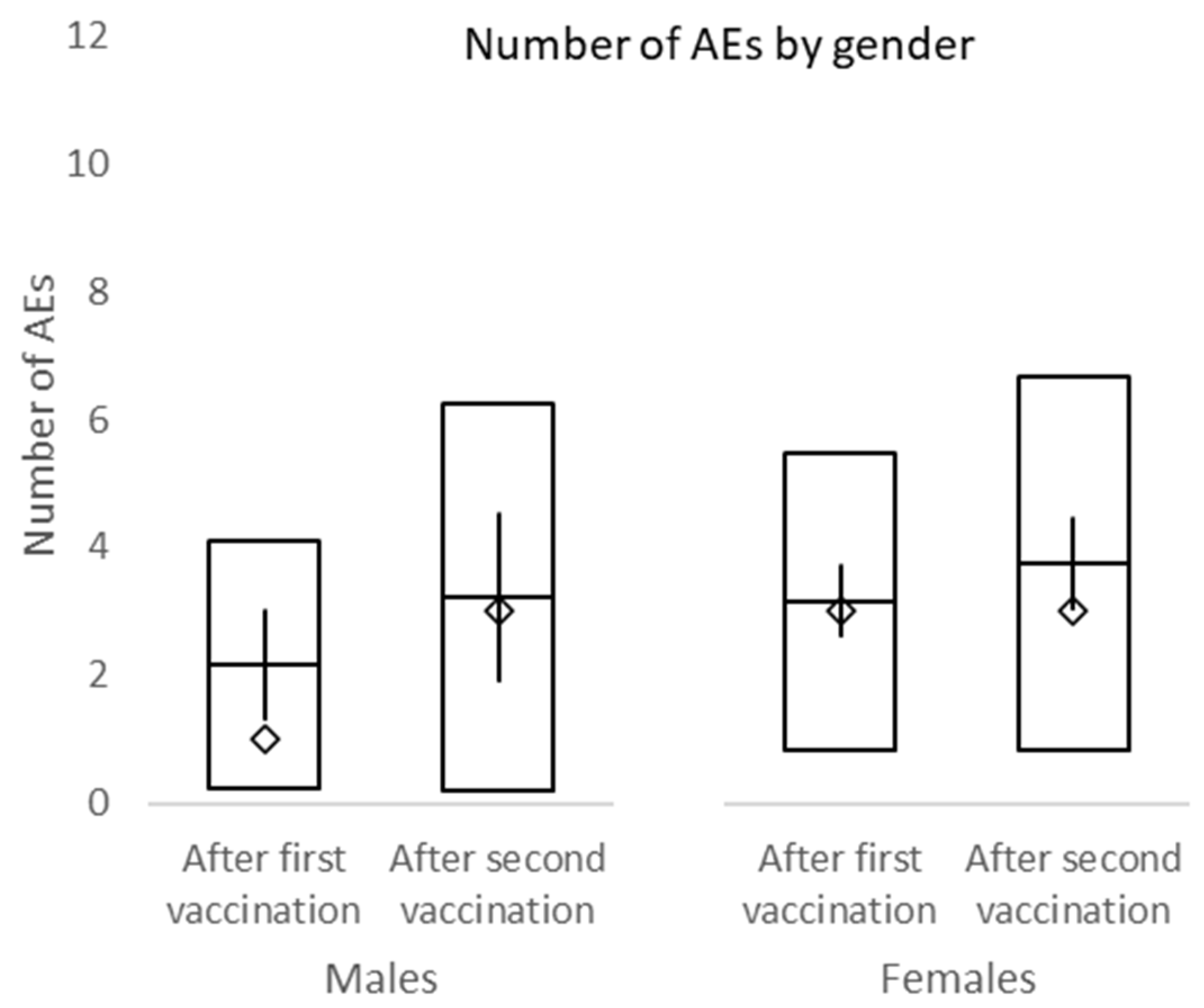
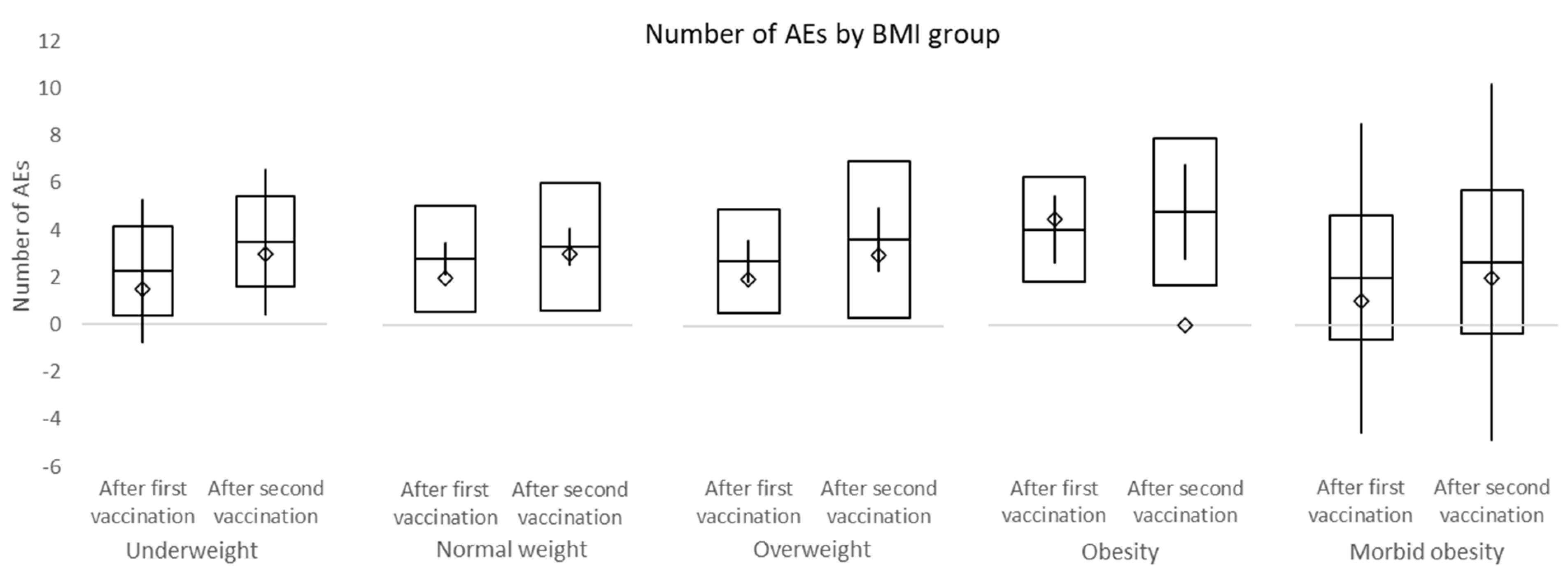
In this study, the overall incidence of adverse events was non-significantly higher after the second dose of vaccine compared to the first dose. No difference in adverse events between male and female and different BMI groups was observed. Among the most frequent side effects of the first vaccine dose were injection site pain (70.2%), limb pain (56.4%), and fatigue (36.2%). The second dose was mostly accompanied by injection site pain (62.8%), fatigue (56.4%), and limb pain (34.0%). The overall incidence of headache after the first and second dose of a vaccine was 16.0% and 26.5%, respectively. No subject within this study required a leave of absence due to adverse events associated with the vaccination. Headache was present in significantly more females than males after both vaccinations. While the incidence of fatigue and fever was significantly higher after the second dose of the vaccine, the incidence of limb pain events declined. No serious adverse events of grade 3/4 were recorded.
Previously, we presented data on a prospective study [
17] with 413 employees (89 men; 306 healthcare professionals) at NCCC with median age of 47 years (range 19–79 years) aimed at exploring the incidence and severity of adverse events after administration of the first dose of BNT162b2. At a median follow-up of 4 weeks, the median number of mRNA vaccine adverse events was significantly higher in females compared to males and healthcare professionals compared to non-healthcare workers. All side effects were mild and no new safety signals were recognized.
Kadali et al. published a randomized, cross-sectional study [
18] performed to explore the adverse events of the BNT162b2 vaccine using an online questionnaire to gather responses from 803 healthcare workers. While the results of this study did differentiate between populations based on age, gender, and level of education, they referred only to adverse events following vaccination without specifying with which dose they were associated. The most common symptoms after vaccination were a sore arm or pain at the injection site (88.04%), fatigue (58.90%), and headache (44.83%). The incidence of both injection site pain and fatigue was comparable with our study, while headache was more often present in this cross-sectional trial.
In our study, we present our results stratified by gender and BMI; moreover, we strictly differentiate between the first and second doses of the vaccine in terms of adverse events. The most common side effects of BNT162b2 were generalized weakness/fatigue and sore arm/pain with similar incidences for each.
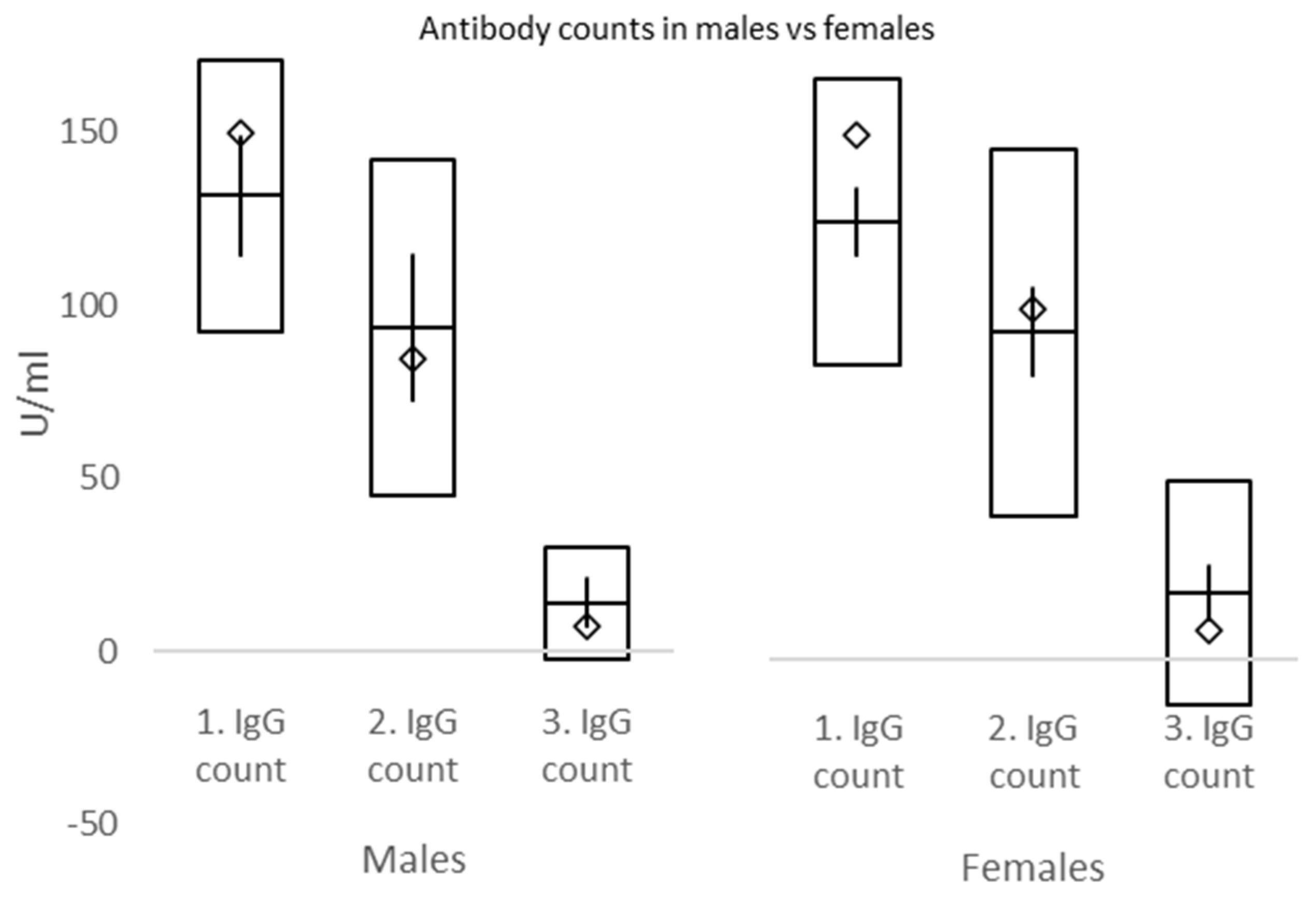
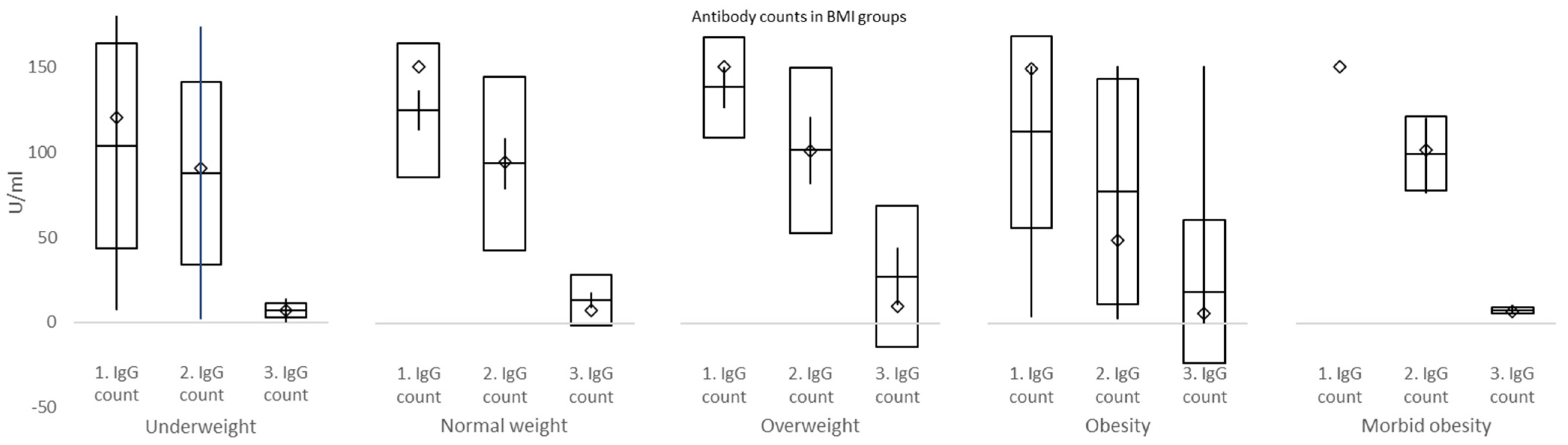
In the current study, IgG counts were measured at 3, 7, and 26 weeks. Significant declines in IgG plasma levels were exposed between weeks 3 and 7, as well as weeks 7 and 26. No differences in IgG between males and females were discovered at week 3, week 7, nor week 26. However, there were significantly higher IgG counts in overweight compared to underweight and morbid obesity vs. normal weight, overweight, and obesity BMI groups at week 3. At week 7, IgG counts were significantly higher in morbid obesity compared to normal weight BMI groups, while at week 26, IgG values in morbidly obese subjects were significantly lower than in normal weight and overweight individuals. These data must be interpreted with caution due to the small number of subjects mostly in morbidly obese and underweight groups. Briefly, our study explored the differences in IgG counts in subjects of varying BMI status at set intervals and confirmed a robust increment followed by a rapid decline of plasma IgG over 6 months after the second dose of BNT162b2.
In a phase I/II study of mRNA vaccine BNT162B1 [
12], only small increases in novel coronavirus–neutralizing geometric mean titers were observed 21 days after the first dose in adults. However, 7 and 14 days after the second dose, substantially greater serum neutralizing geometric mean titers were achieved compared to human convalescent sera. In another study [
13], the antibody responses elicited by BNT162b1 were very similar to those observed in the trial mentioned previously. In summary, BNT162b1 induced robust IgG plasma levels. However, neither analysis assessed immune responses beyond 2 weeks after the second dose of the vaccine.
An assessment of the changes in antibody response up to 6 months after two doses of the BNT162b2 vaccine was recently published by Naaber et al. [
14]. Their findings showed strong Spike receptor binding domain antibody responses 1 week after the second dose, followed by a significant decline at 3 and 6 months afterwards. In older individuals, a weaker antibody response correlating with fewer side effects at the time of vaccinations was recognized.
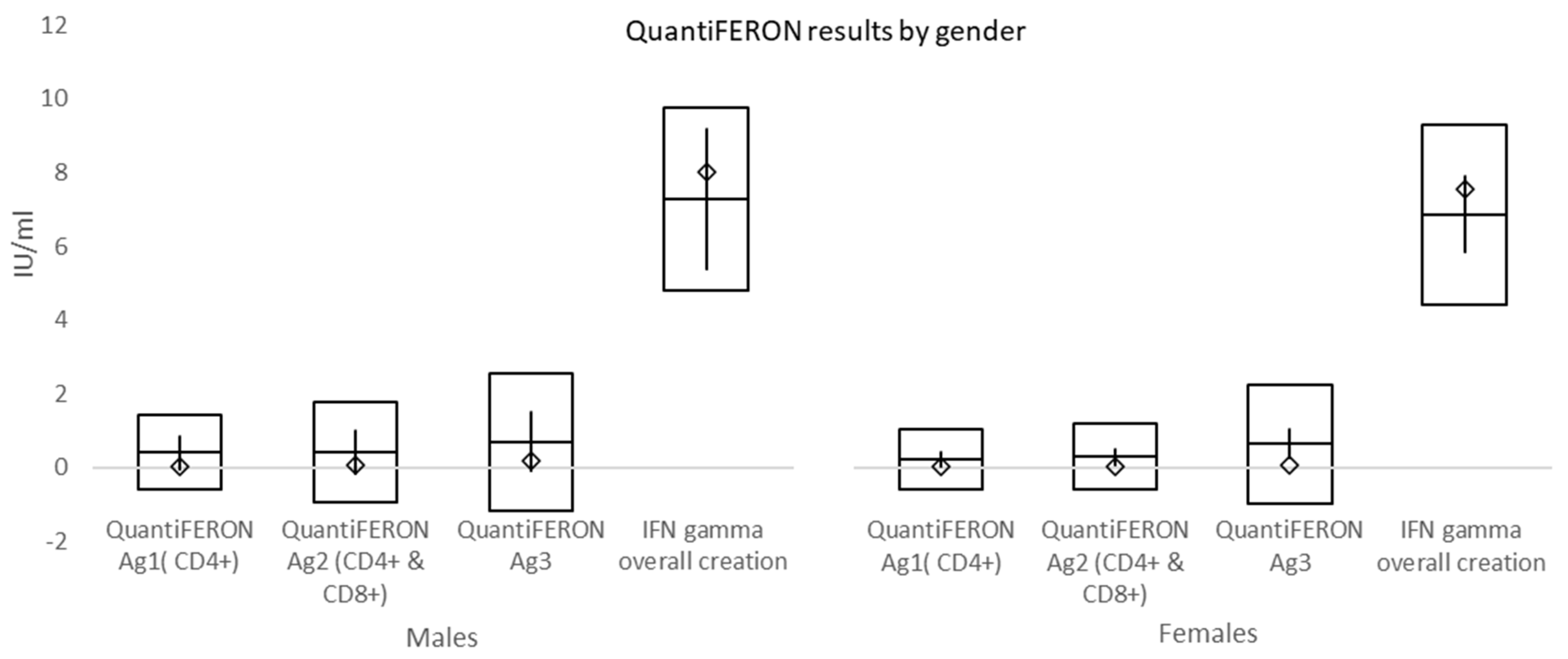
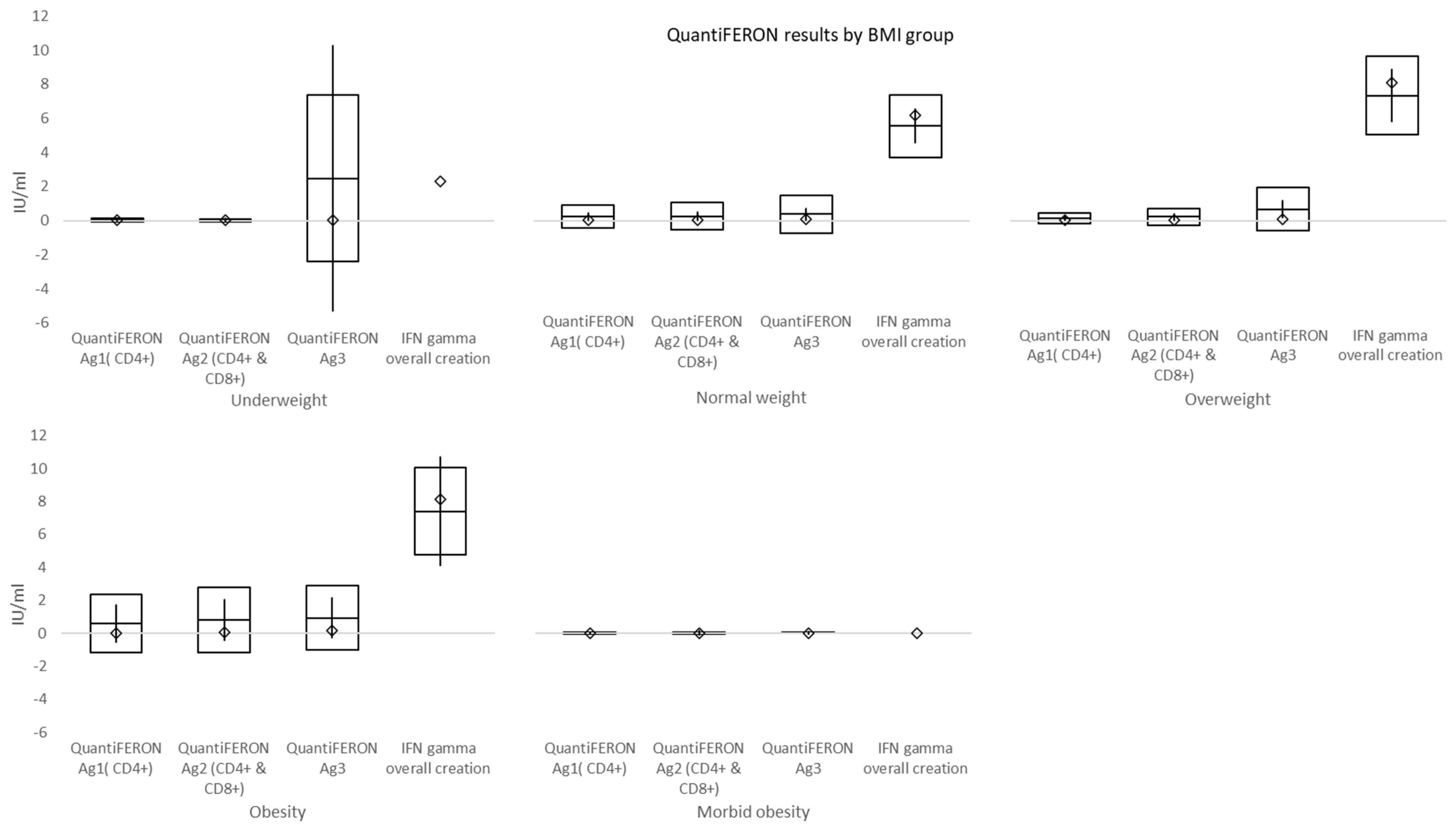
At a mean time of 157 days from double vaccination to immune cell response testing, mean values of QuantiFERON Ag1 activating CD4+ T cells, QuantiFERON Ag2 activating both CD4+ T cells and CD8+ Natural killer cells, and QuantiFERON Ag3 activating CD4+, CD8+, and CD16+ (both T and B cells) were higher than the cut-off (0.15–0.20 IU/mL). Overall interferon-γ (IFN-γ) >10 IU/mL was shown in 63.8% (60/94) of subjects. In individuals with morbid obesity, both QuantiFERON Ag1 and QuantiFERON Ag3 values, reflecting the activation of T cells, were significantly lower when compared to normal weight and overweight subgroups. However, this difference might have been affected by both a small number of individuals and a shorter time interval between the second dose of vaccination and cell-mediated immunity testing in morbidly obese subjects, which was caused by vis major. Overall, positive immune cell response was evident in significantly fewer subjects than borderline and negative responses.
Two doses of BNT162b1 elicited robust CD4
+ and CD8
+ T cell responses. The majority of tested individuals had T helper type 1 (T
H1)-skewed T cell immune responses with RBD-specific CD8
+ and CD4
+ T cell expansion. IFN-γ was produced by a large fraction of RBD-specific T cells (CD8
+ and CD4
+) [
13]. Three months after vaccination with two doses of BNT162b2, 87% of vaccinated individuals developed either CD4+ or CD8+ T cell responses. In addition, CD4+ T cell response was decreased among subjects with elevated senescent CD8+ T cells re-expressing CD45RA (TEMRA) cells [
14].
In conclusion, this prospective study confirmed the high efficacy of the BNT162b2 vaccine (more than 97%) in a specific population of National Comprehensive Cancer Center staff, despite a negative or borderline immune cell response in the majority of individuals and a rapid decline of IgG antibody response 6 months post double vaccination. The decline in antibody responses was more marked in morbidly obese individuals who also had a lower activation of T cells. However, the obtained data must be interpreted with caution due to the small number of subjects in this single-center study, which are its major limitations, and therefore, they must be confirmed in a larger population. Based on this study’s results, we believe that consideration should be given to monitoring immune cells and IgG antibody counts in order to optimize the timing of the administration of booster doses of the BNT162b2 vaccine.