Safety and Immunogenicity of a Booster Vaccination by CoronaVac or BNT162b2 in Previously Two-Dose Inactivated Virus Vaccinated Individuals with Negative Neutralizing Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Automated Chemiluminescent Anti-Spike IgG Assay
2.3. Surrogate Neutralizing Antibody Immunoassay
2.4. T cell-Mediated Immune Response Assay
2.5. Statistics
3. Results
3.1. Demographics and Co-Morbidities
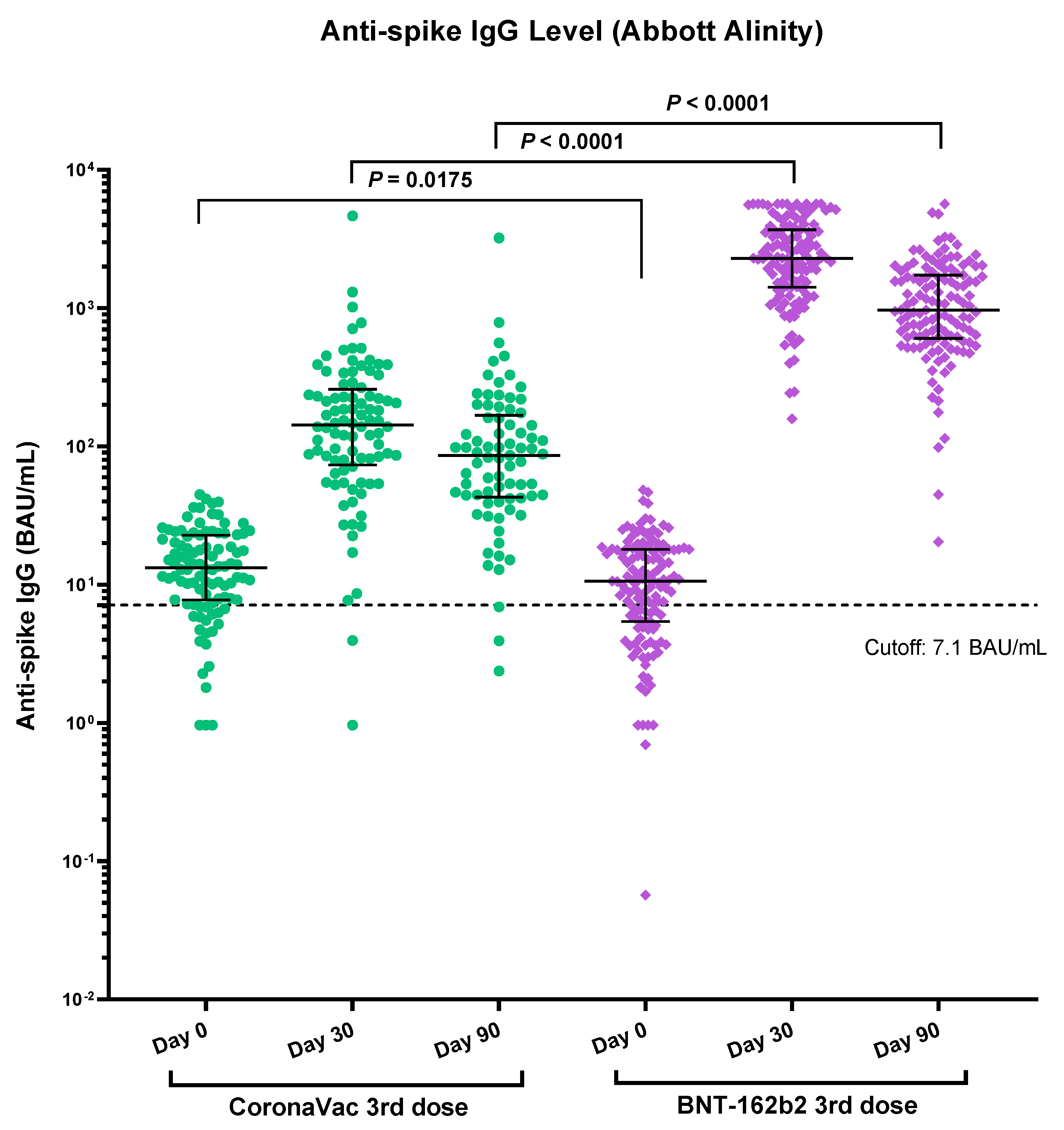
3.2. Immunogenicity of Vaccines Determined by Anti-Spike RBD IgG
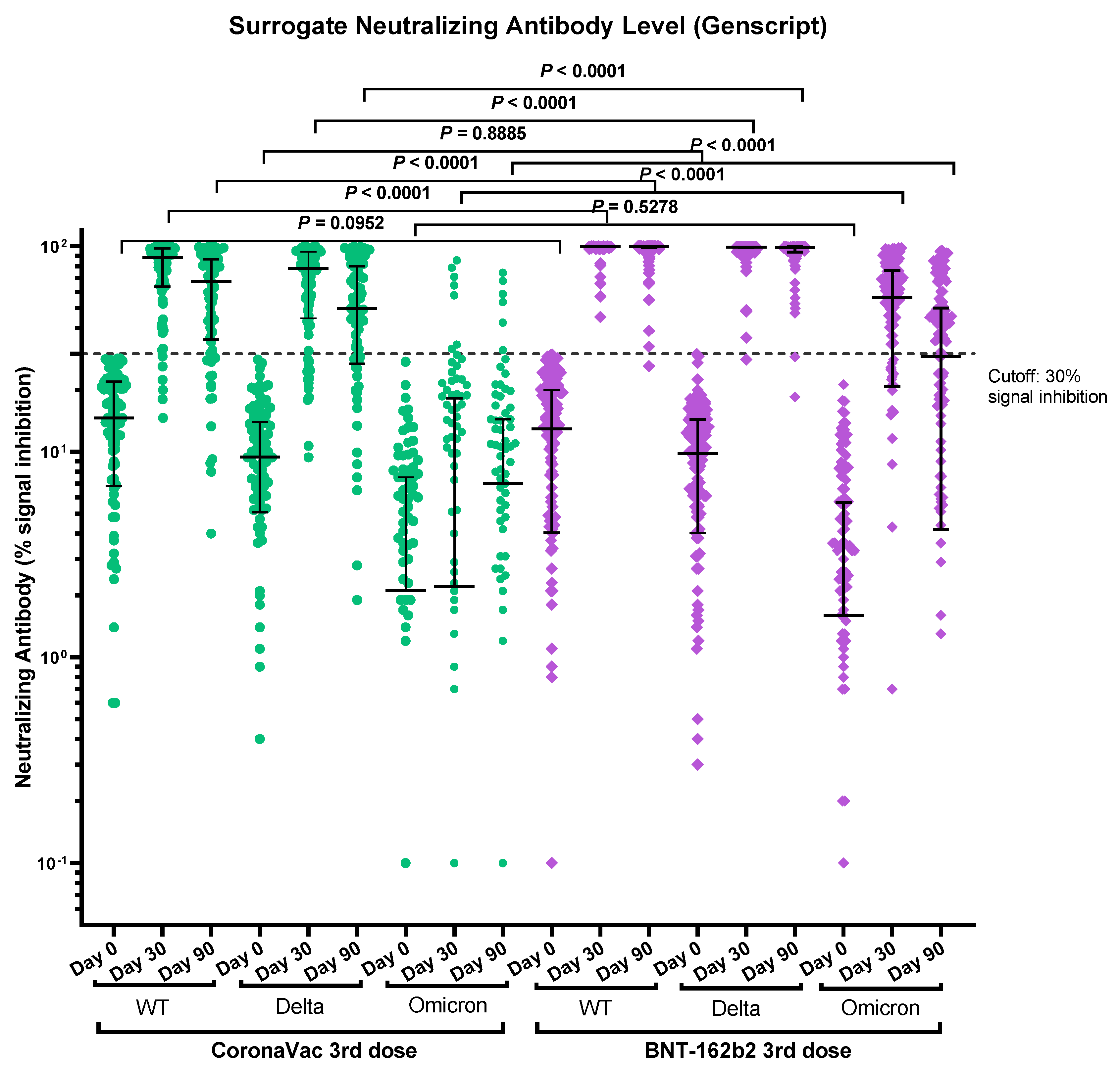
3.3. Immunogenicity of Vaccines Determined by Surrogate Neutralizing Antibody Immunoassay
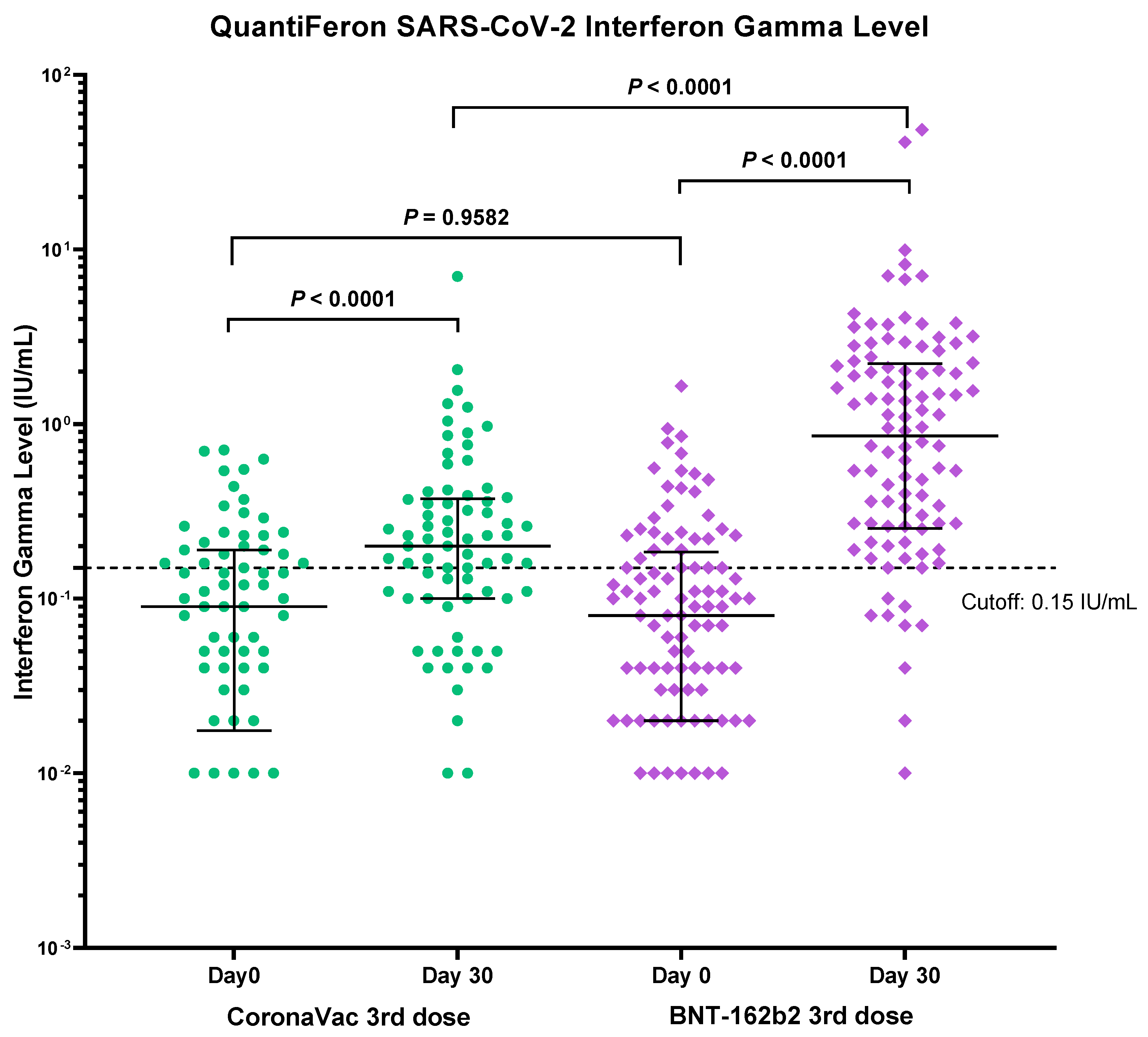
3.4. Immunogenicity of Vaccines Determined by T-cell Mediated Immune Response Assay
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwok, K.O.; Wong, V.; Wei, V.W.I.; Wong, S.Y.S.; Tang, J.W. Novel coronavirus (2019-nCoV) cases in Hong Kong and implications for further spread. J. Infect. 2020, 80, 671–693. [Google Scholar] [CrossRef] [Green Version]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 14 February 2022).
- Center for Health Protection (CHP). Hong Kong Special Administrative Region. CHP Local Situation Dashboard. Available online: https://chp-dashboard.geodata.gov.hk/covid-19/en.html (accessed on 14 February 2022).
- Center for Health Protection (CHP). Hong Kong Special Administrative Region. CHP Vaccination Dashboard. Available online: https://www.covidvaccine.gov.hk/en/dashboard (accessed on 14 February 2022).
- Krüttgen, A.; Klingel, H.; Haase, G.; Haefner, H.; Imohl, M.; Kleines, M. Evaluation of the QuantiFERON SARS-CoV-2 interferon-ɣ release assay in mRNA-1273 vaccinated health care workers. J. Virol. Methods 2021, 298, 114295. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and Cellular Immunogenicity of the BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccine in Nursing Home Residents. Clin. Infect. Dis. 2021, 73, 2145–2147. [Google Scholar] [CrossRef] [PubMed]
- Tychala, A.; Meletis, G.; Katsimpourlia, E.; Gkeka, I.; Dimitriadou, R.; Sidiropoulou, E.; Skoura, L. Evaluation of the QuantiFERON SARS-CoV-2 assay to assess cellular immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in individuals with low and high humoral response. Hum. Vaccin. Immunother. 2021, 29, 1–2. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria 2021. Available online: https://www.R-project.org/ (accessed on 14 February 2022).
- Zee, J.S.T.; Lai, K.T.W.; Ho, M.K.S.; Leung, A.C.P.; Fung, L.H.; Luk, W.P.; Kwok, L.F.; Kee, K.M.; Chan, Q.W.L.; Tang, S.F.; et al. Serological response to mRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: Decline in antibodies 12 weeks after two doses. Hong Kong Med. J. 2021, 27, 380–383. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. RHH-001 study team. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes. Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.F.; Malik, A.A.; De la Cruz, E.; Jorge, A.; et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 3, 481–485. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. DMID 21-0012 Study Group. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J. Med. Virol. 2022, 94, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; You, K.; Lam, B.H.S.; Lau, E.H.Y.; et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022, 3, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Sia, S.F.; Schmitz, A.J.; Bricker, T.L.; Starr, T.N.; Greaney, A.J.; Turner, J.S.; Mohammed, B.M.; Liu, Z.; Choy, K.T.; et al. Neutralizing Monoclonal Antibodies That Target the Spike Receptor Binding Domain Confer Fc Receptor-Independent Protection against SARS-CoV-2 Infection in Syrian Hamsters. mBio 2021, 12, e0239521. [Google Scholar] [CrossRef] [PubMed]
- Glans, H.; Gredmark-Russ, S.; Olausson, M.; Falck-Jones, S.; Varnaite, R.; Christ, W.; Maleki, K.T.; Karlberg, M.L.; Broddesson, S.; Falck-Jones, R.; et al. Shedding of infectious SARS-CoV-2 by hospitalized COVID-19 patients in relation to serum antibody responses. BMC Infect Dis. 2021, 21, 494. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell. Rep. 2021, 34, 108728. [Google Scholar] [CrossRef]
- Chan, K.H.; Leung, K.Y.; Zhang, R.R.; Liu, D.; Fan, Y.; Chen, H.; Yuen, K.Y.; Hung, I.F. Performance of a Surrogate SARS-CoV-2-Neutralizing Antibody Assay in Natural Infection and Vaccination Samples. Diagnostics 2021, 11, 1757. [Google Scholar] [CrossRef]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020, 5, eabd6160. [Google Scholar] [CrossRef] [PubMed]
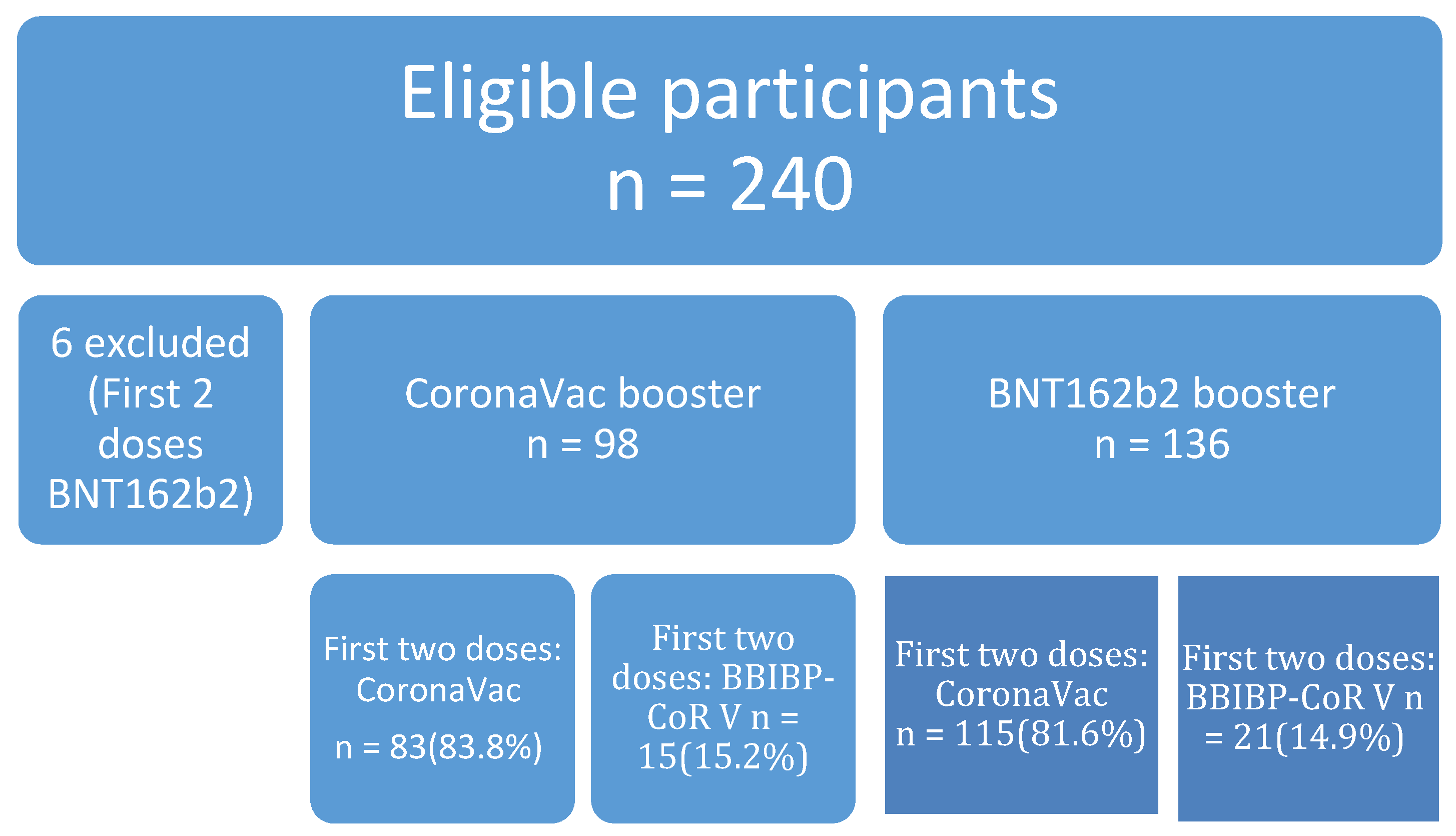
| CoronaVac Group (n = 98) | BNT162b2 Group (n = 136) | p-Value 2 (<65) | p-Value 2 (≥65) | |||
|---|---|---|---|---|---|---|
| Age < 65 (n = 78) | Age ≥ 65 (n = 20) | Age < 65 (n = 93) | Age ≥ 65 (n = 43) | |||
| Female | 42 (53.8%) | 5 (25%) | 53 (57.0%) | 12 (27.9%) | 0.76 | >0.99 |
| Mean age | 49.9 (S.D.9.0) | 70.4 (S.D.3.4) | 52.0 (S.D.7.8) | 70.9 (S.D.5.6) | 0.10 | 0.65 |
| Cardiovascular diseases | 1 (1.3%) | 6 (30.0%) | 1 (1.1%) | 6 (14.0%) | >0.99 | 0.17 |
| Stroke | 0 | 0 | 1 (1.1%) | 0 | >0.99 | >0.99 |
| Hypertension | 16 (20.5%) | 10 (50.0%) | 19 (20.4%) | 20 (46.5%) | >0.99 | >0.99 |
| Diabetes mellitus | 4 (5.1%) | 8 (40.0%) | 6 (6.5%) | 5 (11.6%) | 0.76 | 0.017 |
| Hyperlipidemia | 14 (17.9%) | 6 (30.0%) | 12 (12.9%) | 12 (27.9%) | 0.40 | >0.99 |
| Asthma | 2 (2.6%) | 0 | 0 | 2 (4.7%) | 0.21 | >0.99 |
| Chronic renal diseases | 0 | 0 | 1 (1.1%) | 0 | >0.99 | >0.99 |
| Chronic liver diseases | 2 (2.6%) | 0 | 1 (1.1%) | 0 | 0.59 | >0.99 |
| Cancer | 1 (1.3%) | 0 | 7 (7.5%) | 7 (16.3%) | 0.07 | >0.99 |
| Received chemotherapy and/or radiotherapy | 0 | 0 | 0 | 0 | >0.99 | >0.99 |
| Steroid 3 | 0 | 1 (5.0%) | 3 (3.2%) | 2 (4.7%) | 0.25 | >0.99 |
| AIDS 4 | 0 | 0 | 0 | 0 | >0.99 | >0.99 |
| Systemic lupus erythematosus | 0 | 0 | 1 (1.1%) | 0 | >0.99 | >0.99 |
| Other autoimmune diseases | 1 (1.3%) | 0 | 6 (6.5%) | 2 (4.7%) | 0.12 | >0.99 |
| Food/drug allergy | 0 | 0 | 0 | 0 | >0.99 | >0.99 |
| CoronaVac Group (n = 98) | BNT162b2 Group (n = 136) | p-Value | ||
|---|---|---|---|---|
| Antibody level, BAU/mL, median (IQR *) | Baseline | 13.2 (7.8–22.8) n = 98 | 10.6 (5.4–18.0) n = 136 | <0.0175 |
| Day 30 | 143 (73.4–259) n = 92 | 2302 (1414–3677) n = 130 | <0.0001 | |
| Day 90 | 86.1 (43.0–167.8) n = 77 | 968 (604.2–1740) n = 126 | <0.0001 | |
| NAb positivity against wild type | Day 30 | 90% (83/92) | 100% (130/130) | 0.0003 |
| Day 90 | 79% (61/77) | 99% (125/126) | <0.0001 | |
| NAb positivity against Delta | Day 30 | 82% (75/92) | 99% (129/130) | <0.0001 |
| Day 90 | 70% (54/77) | 97% (122/126) | <0.0001 | |
| NAb positivity against Omicron | Day 30 | 7% (6/92) | 69% (90/130) | <0.0001 |
| Day 90 | 6% (5/77) | 48% (61/126) | <0.0001 |
| CoronaVac Group (n = 98) | Decline Rate | Fold Change | BNT162b2 Group (n = 136) | Decline Rate | Fold Change | ||
|---|---|---|---|---|---|---|---|
| NAb Median percentage inhibition against WT | Day 30 | 87.9 (63.6–97.7) n = 92 | Day 30 to 90: −23.5% | 99.5 (99.3–99.6) n = 130 | Day 30 to 90: 0.0% | ||
| Day 90 | 67.2 (35.2–86.5) n = 77 | 99.5 (98.7–99.6) n = 126 | |||||
| NAb Median percentage inhibition against Delta | Day 30 | 78.2 (44.6–94.1) n = 92 | Day 30 to 90: −36.4% | Delta Day 30 vs. Omicron Day 30: 35.5 | 99.1 (98.3–99.3) n = 130 | Day 30 to 90: −0.3% | Delta Day 30 vs. Omicron Day 30: 1.8 |
| Day 90 | 49.7 (26.8–80.2) n = 77 | 98.8 (93.6–99.4) n = 126 | |||||
| NAb Median percentage inhibition against Omicron | Day 30 | 2.2 (0–18.2) n = 92 | Day 30 to 90: 218.2% * | Delta Day 90 vs. Omicron Day 90: 7.1 | 56.4 (20.9–75.9) n = 130 | Day 30 to 90: −48.4% | Delta Day 90 vs. Omicron Day 90: 3.4 |
| Day 90 | 7 (0–14.4) n = 77 | 29.1 (4.2–50.1) n = 126 |
| CoronaVac Group (n = 74) | BNT162b2 Group (n = 104) | p-Value 1 | |
|---|---|---|---|
| Positive at baseline | 33.8 % (25/74) | 30.8% (32/104) | p = 0.9582 |
| Positive at day 30 | 63.5% (47/74) | 86.5% (90/104) | p < 0.0001 |
| CoronaVac Group (n = 98) | BNT162b2 Group (n = 136) | p-Value | |
|---|---|---|---|
| No data | 10.2% (10/98) | 5.0% (7/136) | |
| No adverse effect | 74.5% (73/98) | 47.8% (65/136) | |
| Any adverse effect | 16.3% (16/98) | 50.7% (69/136) | <0.0001 |
| Fever | 4.5% (4/88) | 14.7% (19/129) | 0.0230 |
| Chills | 8.0% (7/88) | 7.0% (9/129) | 0.7967 |
| Injection site pain | 13.6% (12/88) | 39.5% (51/129) | <0.0001 |
| Fatigue | 6.8% (6/88) | 32.6% (42/129) | <0.0001 |
| Headache | 8.0% (7/88) | 14.0% (18/129) | 0.1995 |
| Muscle pain | 2.3% (2/88) | 34.9% (45/129) | <0.0001 |
| Diarrhoea | 2.3% (2/88) | 5.4% (7/129) | 0.3170 |
| Joint pain | 1.1% (1/88) | 14.7% (19/129) | 0.0005 |
| Skin rash | 2.3% (2/88) | 3.9% (5/129) | 0.7037 |
| Nausea/vomiting | 1.1% (1/88) | 2.3% (3/129) | 0.6483 |
| Tremor | 0 | 1.6% (2/129) | 0.5156 |
| Abdominal pain | 0 | 0.8% (1/129) | >0.99 |
| Urticaria | 0 | 1.6% (2/129) | 0.5156 |
| Enlarged lymph nodes | 0 | 1.6% (2/129) | 0.5156 |
| Sore throat | 0 | 3.9% (5/129) | 0.0821 |
| Nasal congestion | 0 | 5.4% (7/129) | 0.0432 |
| SAE 1 | 0 | 0 | >0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, K.T.-W.; Lai Wan Loong, E.Y.-T.; Fung, T.L.-H.; Luk, L.W.-P.; Lau, C.-C.; Zee, J.S.-T.; Ma, E.S.-K.; Tang, B.S.-F. Safety and Immunogenicity of a Booster Vaccination by CoronaVac or BNT162b2 in Previously Two-Dose Inactivated Virus Vaccinated Individuals with Negative Neutralizing Antibody. Vaccines 2022, 10, 556. https://doi.org/10.3390/vaccines10040556
Lai KT-W, Lai Wan Loong EY-T, Fung TL-H, Luk LW-P, Lau C-C, Zee JS-T, Ma ES-K, Tang BS-F. Safety and Immunogenicity of a Booster Vaccination by CoronaVac or BNT162b2 in Previously Two-Dose Inactivated Virus Vaccinated Individuals with Negative Neutralizing Antibody. Vaccines. 2022; 10(4):556. https://doi.org/10.3390/vaccines10040556
Chicago/Turabian StyleLai, Kristi Tsz-Wan, Emilie Yuen-Ting Lai Wan Loong, Terry Ling-Hiu Fung, Luke Wing-Pan Luk, Chor-Chiu Lau, Jonpaul Sze-Tsing Zee, Edmond Shiu-Kwan Ma, and Bone Siu-Fai Tang. 2022. "Safety and Immunogenicity of a Booster Vaccination by CoronaVac or BNT162b2 in Previously Two-Dose Inactivated Virus Vaccinated Individuals with Negative Neutralizing Antibody" Vaccines 10, no. 4: 556. https://doi.org/10.3390/vaccines10040556
APA StyleLai, K. T.-W., Lai Wan Loong, E. Y.-T., Fung, T. L.-H., Luk, L. W.-P., Lau, C.-C., Zee, J. S.-T., Ma, E. S.-K., & Tang, B. S.-F. (2022). Safety and Immunogenicity of a Booster Vaccination by CoronaVac or BNT162b2 in Previously Two-Dose Inactivated Virus Vaccinated Individuals with Negative Neutralizing Antibody. Vaccines, 10(4), 556. https://doi.org/10.3390/vaccines10040556







