Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Clinical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.C.; Shah, A.; Sah, P.; Moghadas, S.M.; Vilches, T.; Galvani, A.P. The U.S. COVID-19 Vaccination Program at One Year: How Many Deaths and Hospitalizations Were Averted? Available online: https://www.commonwealthfund.org/publications/issue-briefs/2021/dec/us-COVID-19-vaccination-program-one-year-how-many-deaths-and (accessed on 7 March 2022).
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody Titers against SARS-CoV-2 Decline, but Do Not Disappear for Several Months. Eclinical Med. 2021, 32, 100734. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Shin, B.-H.; Gadsden, T.-A.M.; Chu, M.; Petrosyan, A.; Le, C.N.; Zabner, R.; Oft, J.; Pedraza, I.; Cheng, S.; et al. T Cell Immune Responses to SARS-CoV-2 and Variants of Concern (Alpha and Delta) in Infected and Vaccinated Individuals. Cell. Mol. Immunol. 2021, 18, 2554–2556. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. mRNA Vaccine-Elicited SARS-CoV-2-Specific T Cells Persist at 6 Months and Recognize the Delta Variant. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Rzymski, P.; Camargo, C.A.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 Booster Vaccines against COVID-19 Related Symptoms, Hospitalisation and Death in England. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 Vaccine Breakthrough: Clinical Characteristics of 152 Fully Vaccinated Hospitalized COVID-19 Patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Hallam, J.; Jones, T.; Alley, J.; Kohut, M.L. Exercise after Influenza or COVID-19 Vaccination Increases Serum Antibody without an Increase in Side Effects. Brain Behav. Immun. 2022, 102, 1–10. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Simon, K.; Łapiński, T.; Zarębska-Michaluk, D.; Szczepańska, B.; Chojnicki, M.; Mozer-Lisewska, I.; Flisiak, R. Clinical Characteristics of Hospitalized COVID-19 Patients Who Received at Least One Dose of COVID-19 Vaccine. Vaccines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Rogalska, M.; Rorat, M.; Czupryna, P.; Lorenc, B.; Ciechanowski, P.; Kozielewicz, D.; Piekarska, A.; et al. Demographic and Clinical Overview of Hospitalized COVID-19 Patients during the First 17 Months of the Pandemic in Poland. J. Clin. Med. 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R Mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital Admission and Emergency Care Attendance Risk for SARS-CoV-2 Delta (B.1.617.2) Compared with Alpha (B.1.1.7) Variants of Concern: A Cohort Study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html (accessed on 31 January 2022).
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Our World Data. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 24 March 2022).
- Mahat, R.K.; Panda, S.; Rathore, V.; Swain, S.; Yadav, L.; Sah, S.P. The Dynamics of Inflammatory Markers in Coronavirus Disease-2019 (COVID-19) Patients: A Systematic Review and Meta-Analysis. Clin. Epidemiol. Glob. Health 2021, 11, 100727. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 26 April 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Diagnosis and Therapy of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 12 November 2021. Annex No. 1 to the Recommendations of 26 April 2021. Pol. Arch. Intern. Med. 2021, 131. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta Variant Replication and Immune Evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Aguiar, M.; Dosi, G.; Knopoff, D.A.; Virgillito, M.E. A Multiscale Network-Based Model of Contagion Dynamics: Heterogeneity, Spatial Distancing and Vaccination. Math. Models Methods Appl. Sci. 2021, 31, 2425–2454. [Google Scholar] [CrossRef]
- Sikora, D.; Rzymski, P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines 2022, 10, 437. [Google Scholar] [CrossRef]
- Rzymski, P.; Borkowski, L.; Drąg, M.; Flisiak, R.; Jemielity, J.; Krajewski, J.; Mastalerz-Migas, A.; Matyja, A.; Pyrć, K.; Simon, K.; et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Colagiuri, B. Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines. medRxiv 2022. [Google Scholar] [CrossRef]
- Raport Szczepień Przeciwko COVID-19—Szczepienie Przeciwko COVID-19—Portal Gov.pl. Available online: https://www.gov.pl/web/szczepimysie/raport-szczepien-przeciwko-COVID-19 (accessed on 24 March 2022).
- Zarębska-Michaluk, D.; Rzymski, P.; Moniuszko-Malinowska, A.; Brzdęk, M.; Martonik, D.; Rorat, M.; Wielgat, J.; Kłos, K.; Musierowicz, W.; Wasilewski, P.; et al. Does Hospitalization Change the Perception of COVID-19 Vaccines among Unvaccinated Patients? Vaccines 2022, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Horeanga, A.; Papleux, E.; Vekemans, M.; Beukinga, I.; Blairon, L. Immunogenicity of MRNA-1273 COVID Vaccine after 6 Months Surveillance in Health Care Workers: A Third Dose Is Necessary. J. Infect. 2021, 83, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Marking, U.; Havervall, S.; Greilert-Norin, N.; Ng, H.; Blom, K.; Nilsson, P.; Phillipson, M.; Hober, S.; Nilsson, C.; Mangsbo, S.; et al. Duration of SARS-CoV-2 Immune Responses up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 NCoV-19 and BNT162b2 MRNA Vaccines. Vaccines 2022, 10, 359. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by COVID-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Aghayari Sheikh Neshin, S.; Khatami, A.; Turner, D.L.; Djalalinia, S.; Mousavi, S.A.; Mardani-Fard, H.A.; et al. Effectiveness of COVID-19 Vaccines against Delta (B.1.617.2) Variant: A Systematic Review and Meta-Analysis of Clinical Studies. Vaccines 2021, 10, 23. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of MRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological Mechanisms of Vaccine-Induced Protection against COVID-19 in Humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against COVID-19 by BNT162b2 Booster across Age Groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 Doses of MRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.S.; Ogun, O.A.; Simmons, S.R.; Zamparo, J.M.; et al. Effectiveness of a Third Dose of BNT162b2 mRNA COVID-19 Vaccine in a Large US Health System: A Retrospective Cohort Study. Lancet Reg. Health Am. 2022, 100198. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of MRNA Vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar]
- Rzymski, P.; Poniedziałek, B.; Fal, A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines 2021, 9, 1286. [Google Scholar] [CrossRef]
- Gavenčiak, T.; Monrad, J.T.; Leech, G.; Sharma, M.; Mindermann, S.; Brauner, J.M.; Bhatt, S.; Kulveit, J. Seasonal Variation in SARS-CoV-2 Transmission in Temperate Climates. medRxiv 2021. [Google Scholar] [CrossRef]
- Tixagevimab and Cilgavimab (Evusheld) for Pre-Exposure Prophylaxis of COVID-19. JAMA 2022, 327, 384–385. [CrossRef]
- Zhou, H.; Tada, T.; Dcosta, B.M.; Landau, N.R. SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies. bioRxivorg 2022. [Google Scholar] [CrossRef]
- Maruggi, G.; Mallett, C.P.; Westerbeck, J.W.; Chen, T.; Lofano, G.; Friedrich, K.; Qu, L.; Sun, J.T.; McAuliffe, J.; Kanitkar, A.; et al. A Self-Amplifying MRNA SARS-CoV-2 Vaccine Candidate Induces Safe and Robust Protective Immunity in Preclinical Models. Mol. Ther. 2022. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-Dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef]
- Faro-Viana, J.; Bergman, M.-L.; Gonçalves, L.A.; Duarte, N.; Coutinho, T.P.; Borges, P.C.; Diwo, C.; Castro, R.; Matoso, P.; Malheiro, V.; et al. Population Homogeneity for the Antibody Response to COVID-19 BNT162b2/Comirnaty Vaccine Is Only Reached after the Second Dose across All Adult Age Ranges. Nat. Commun. 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Ursini, F.; Gragnani, L.; Raimondo, V.; Giuggioli, D.; Foti, R.; Caminiti, M.; Olivo, D.; Cuomo, G.; Visentini, M.; et al. Impaired Immunogenicity to COVID-19 Vaccines in Autoimmune Systemic Diseases. High Prevalence of Non-Response in Different Patients’ Subgroups. J. Autoimmun. 2021, 125, 102744. [Google Scholar] [CrossRef] [PubMed]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and Humoral Response after MRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients. Am. J. Transpl. 2021, 21, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
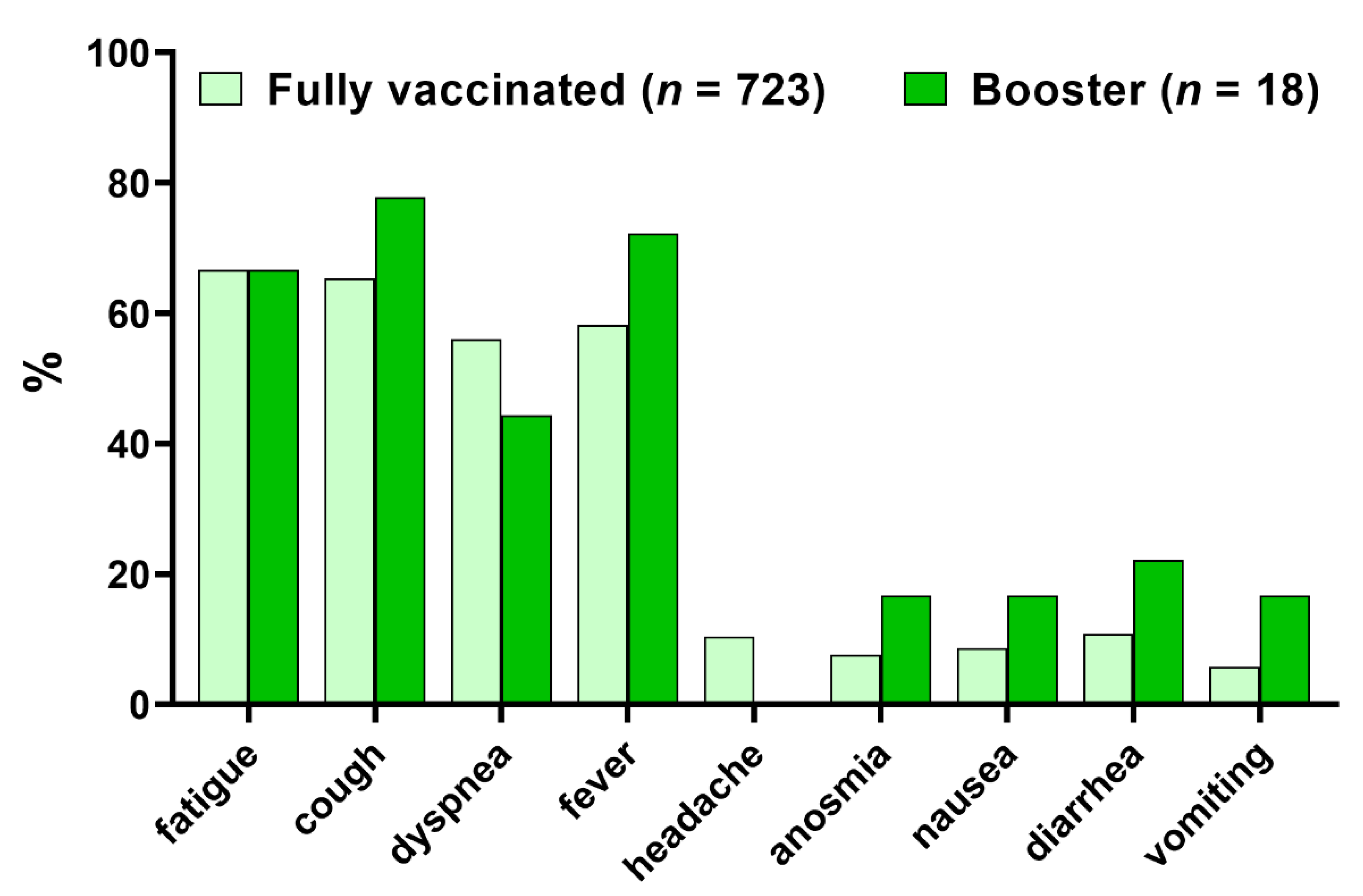
| Parameter | Fully Vaccinated | Booster Dose | p-Value |
|---|---|---|---|
| (n = 723) | (n = 18) | ||
| Age | |||
| Mean ± SD, years | 68.2 ± 15.8 | 73.9 ± 16.6 | ns |
| Range, years | 18–99 | 25–91 | |
| >70 years, % (n) | 49.5 (358) | 72.2 (13) | ns |
| Female/Male, % (n) | 43.9 (317)/56.1 (406) | 33.3 (6)/66.7 (12) | ns |
| BMI | |||
| Mean ± SD, kg/m2 | 28.6 ± 5.7 | 25.4 ± 3.0 | <0.05 |
| Range, kg/m2 | 15.6–54.9 | 19.5–31.0 | |
| Obesity (≥30.0), % (n) | 26.6 (192) | 11.1 (2) | ns |
| Comorbidities, % (n) | 84.9 (614) | 94.4 (17) | ns |
| Asthma, % (n) | 7.6 (55) | 16.7 (3) | ns |
| Cardiovascular, % (n) | 73.9 (534) | 83.3 (15) | ns |
| Cancer, % (n) | 12.7 (92) | 16.7 (3) | ns |
| Chronic kidney disease, % (n) | 18.3 (132) | 33.3 (6) | ns |
| Diabetes, % (n) | 30.4 (220) | 38.9 (7) | ns |
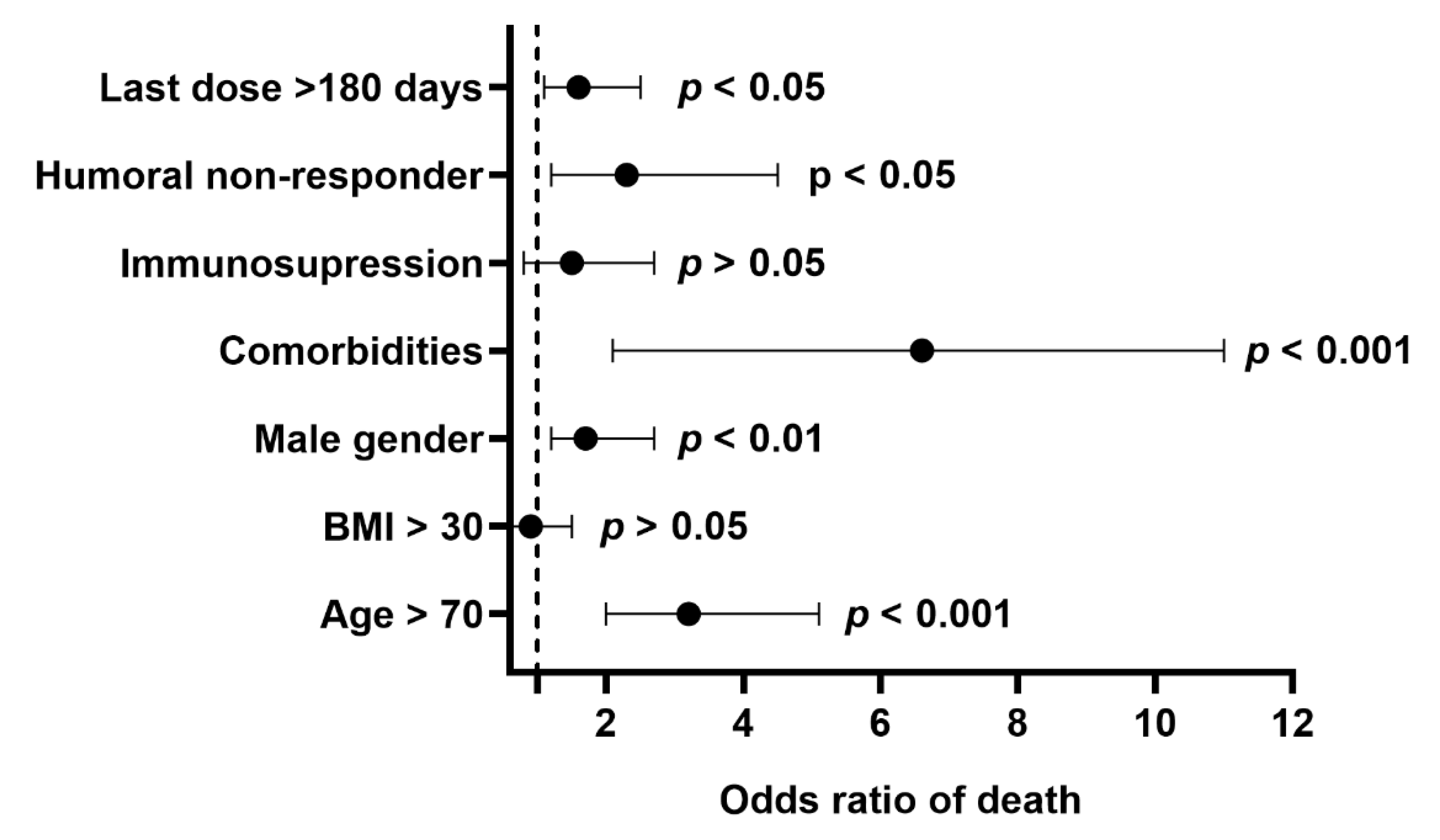
| Immunosuppression, % (n) | 11.1 (80) | 33.3 (6) | <0.01 |
| Non-responders, % (n) | 13.0 (94) | 11.1 (2) | ns |
| Days from the last vaccine dose | |||
| Mean ± SD (range) | 181.1 ± 56.3 (15–329) | 56.3 ± 70.0 (15–321) * | - |
| >180 days, % (n) | 50.2 (362) | - |
| Parameter | Fully Vaccinated (n = 723) | Booster Dose (n = 18) | p-Value |
|---|---|---|---|
| ALC *, ×103/µL, mean ± SD | 1.2 ± 1.4 a | 1.0 ± 0.7 | ns |
| ANC, ×103/µL, mean ± SD | 6.0 ± 6.1 | 8.0 ± 7.4 | ns |
| NLR *, mean ± SD | 7.3 ± 7.2 | 8.4± 7.3 | ns |
| PLC, ×103/µL, mean ± SD | 211.9 ± 99.4 | 171.7 ± 50.3 | ns |
| WBC, ×103/µL, mean ± SD | 8.3 ± 9.2 | 8.9 ± 7.0 | ns |
| >11 × 103/µL, % (n) | 14.6 (106) | 11.1 (2) | ns |
| CRP, mg/L, mean ± SD | 85.8 ± 81.2 | 75.7 ± 60.2 | ns |
| >100 mg/L, % (n) | 34.0 (246) | 2.8 (5) | ns |
| IL-6, pg/mL, mean ± SD | 109.3 ± 357.3 | 55.7 ± 69.5 | ns |
| >100 pg/mL, % (n) | 13.4 (97) | 5.5 (1) | ns |
| PCT, ng/mL, mean ± SD | 3.6 ± 16.9 | 0.1 ± 0.2 | ns |
| >0.25 ng/mL, % (n) | 23.2 (168) | 11.1 (2) | ns |
| Received vaccine | - | ||
| BNT162b2, % (n) | 64.0 (463) | 100.0 (18) | |
| mRNA-1273, % (n) | 7.1 (51) | ||
| AZD1222, % (n) | 18.8 (136) | ||
| AD26.COV2.S, % (n) | 9.4 (68) | ||
| Heterologous, % (n) | 0.7 (4) | ||
| d-dimer, ng/mL, mean ± SD | 2031.1 ± 7144.2 | 1193.6 ± 1492.9 | ns |
| Hospital stay, days | 11.8 ± 8.6 | 12.5 ± 7.4 | ns |
| Baseline WHO, median, (IQR) | 4 (4–5) | 4 (4–4) | ns |
| Admission SpO2, % | 90.0 ± 7.9 | 92 ± 4.3 | ns |
| Lung involvement, % | 27.7 ± 21.5 | 28.4 ± 22.8 | ns |
| Oxygen therapy, % (n) | 81.2 (587) | 11.1 (2) | ns |
| Mechanical ventilation, % (n) | 4.0 (29) | 5.5 (1) | ns |
| Parameter | Fully Vaccinated | Booster Dose | p-Value |
|---|---|---|---|
| (n = 98) | (n = 4) | ||
| Age | |||
| Mean ± SD, years | 78.7 ± 10.6 | 73.2 ± 13.6 | ns |
| >70 years, % (n) | 74.5 (73) | 50.0 (2) | ns |
| Female/Male, % (n) | 32.6 (32)/67.4 (66) | 25.0 (1)/75.0 (3) | ns |
| BMI | |||
| Mean ± SD, kg/m2 | 27.9 ± 6.3 | 25.5 ± 2.5 | ns |
| Obesity (≥30.0), % (n) | 19.4 (19) | 0.0 (0) | ns |
| Comorbidities, % (n) | 96.9 (95) | 100 (4) | ns |
| Asthma, % (n) | 5.1 (5) | 25.0 (1) | ns |
| Cardiovascular, % (n) | 87.7 (86) | 75.0 (3) | ns |
| Cancer, % (n) | 73.5 (72) | 0.0 (0) | ns |
| Chronic kidney disease, % (n) | 33.7 (33) | 25.0 (1) | ns |
| Diabetes, % (n) | 36.7 (36) | 50.0 (2) | ns |
| Immunosuppression, % (n) | 14.3 (14) | 50.0 (2) | ns |
| Humoral non-responders, % (n) | 16.3 (16) | 25.0 (1) | ns |
| Days from the last vaccine dose | - | ||
| Mean ± SD (range) | 194.4 ± 53.9 (72–303) | 50.5 ± 27.3 (17–83) | |
| >180 days, % (n) | 58.2 (57) | - | |
| ALC *, ×103/µL, mean ± SD | 1.0 ± 1.9 | 0.5 ± 0.8 | ns |
| ANC, ×103/µL, mean ± SD | 8.7 ± 12.6 | 29.4 ± 42.3 | <0.05 |
| NLR *, mean ± SD | 21.8 ± 95.6 | 56.2 ± 81.5 | ns |
| PLC, ×103/µL, mean ± SD | 191.7 ± 99.4 | 144.8 ± 38.2 | ns |
| WBC, ×103/µL, mean ± SD | 10.2 ± 11.8 | 11.4 ± 9.2 | ns |
| >11 × 103/µL, % (n) | 27.6 (27) | 25.0 (1) | ns |
| CRP, mg/L, mean ± SD | 128.8 ± 93.2 | 93.1 ± 36.1 | ns |
| >100 mg/L, % (n) | 57.1 (56) | 50 (2) | ns |
| IL-6, pg/mL, mean ± SD | 283.2 ± 618.1 | 105.4 ± 119.9 | ns |
| >100 pg/mL, % (n) | 26.5 (26) | 25.0 (1) | ns |
| PCT, ng/mL, mean ± SD | 3.0 ± 11.9 | 0.3 ± 0.3 | <0.01 |
| >0.25 ng/mL, % (n) | 48.0 (47) | 25.0 (1) | ns |
| d-dimer, ng/mL, mean ± SD | 4371.1 ± 14900 | 1550.2 ± 2423.9 | ns |
| Baseline WHO, median (IQR) | 4 (4–5) | 4 (4–4) | ns |
| Admission SpO2, % | 84.0 ± 13.8 | 83.5 ± 4.5 | ns |
| Lung involvement, % | 40.9 ± 25.8 | 53.7 ±26.9 | ns |
| Oxygen therapy, % (n) | 94.5 (93) | 100.0 (4) | ns |
| Mechanical ventilation,% (n) | 25.5 (25) | 25.0 (1) | ns |
| Parameter | ≤180 Days (n = 361) | >180 Days (n = 362) | p-Value |
|---|---|---|---|
| Age | |||
| Mean ± SD, years | 63.6 ± 15.2 | 72.7 ± 14.9 | <0.001 |
| Range, years | 18–93 | 19–99 | |
| >70 years, % (n) | 33.5 (121) | 65.5 (237) | <0.001 |
| Female/Male, % (n) | 42.9 (155)/57.1 (206) | 44.7 (162)/55.3 (200) | ns |
| BMI | |||
| Mean ± SD, kg/m2 | 28.8 ± 5.8 | 28.3 ± 5.6 | ns |
| Range, kg/m2 | 15.6–54.9 | 17.6–49.0 | |
| Obesity (≥30.0), % (n) | 28.5 (103) | 24.6 (89) | ns |
| Comorbidities, % (n) | 79.5 (287) | 90.3 (327) | <0.001 |
| Asthma, % (n) | 6.9 (25) | 8.3 (30) | ns |
| Cardiovascular, % (n) | 69.0 (249) | 78.7 (285) | <0.01 |
| Cancer, % (n) | 11.6 (42) | 13.8 (50) | ns |
| Chronic kidney disease, % (n) | 15.0 (54) | 21.6 (78) | ns |
| Diabetes, % (n) | 29.4 (106) | 31.5 (114) | ns |
| Immunosuppression, % (n) | 11.1 (40) | 11.0 (40) | ns |
| Humoral non-responders, % (n) | 12.4 (45) | 13.5 (13.5) | ns |
| ALC *, ×103/µL, mean ± SD | 1.5 ± 4.9 | 1.6 ± 5.9 | ns |
| ANC, ×103/µL, mean ± SD | 5.7 ± 3.3 | 6.2 ± 5.9 | ns |
| NLR *, mean ± SD | 7.4 ± 7.9 | 9.6 ± 5.2 | ns |
| PLC, ×103/µL, mean ± SD | 215.9 ± 99.5 | 207.9 ± 99.4 | ns |
| WBC, ×103/µL, mean ± SD | 8.4 ± 9.7 | 8.2 ± 8.7 | ns |
| >11 × 103/µL, % (n) | 15.5 (56) | 13.8 (50) | ns |
| CRP, mg/L, mean ± SD | 90.3 ± 87.0 | 81.2 ± 74.8 | ns |
| >100 mg/L, % (n) | 37.2 (134) | 31.0 (112) | ns |
| IL-6, pg/mL, mean ± SD | 110.4 ± 405.4 | 108.0 ± 294.7 | ns |
| >100 pg/mL, % (n) | 13.3 (48) | 13.5 (49) | ns |
| PCT, ng/mL, mean ± SD | 3.5 ± 17.1 | 3.4 ± 16.8 | ns |
| >0.25 ng/mL, % (n) | 23.5 (85) | 22.9 (83) | ns |
| D-dimer, ng/mL, mean ± SD | 1504.4 ± 3472.7 | 2563.8 ± 9489.1 | <0.05 |
| Hospital stay, days | 11.5 ± 7.7 | 12.1 ± 9.3 | ns |
| Baseline WHO, median, (IQR) | 4 (4–5) | 4 (4–5) | ns |
| Admission SpO2, % | 90.1 ± 7.3 | 89.9 ± 8.5 | ns |
| Lung involvement, % | 27.0 ± 21.5 | 28.4 ± 21.4 | ns |
| Oxygen therapy, % (n) | 79.5 (287) | 82.9 (300) | ns |
| Mechanical ventilation, % (n) | 4.7 (17) | 3.3 (12) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. https://doi.org/10.3390/vaccines10040557
Rzymski P, Pazgan-Simon M, Kamerys J, Moniuszko-Malinowska A, Sikorska K, Wernik J, Zarębska-Michaluk D, Supronowicz Ł, Sobala-Szczygieł B, Skrzat-Klapaczyńska A, et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines. 2022; 10(4):557. https://doi.org/10.3390/vaccines10040557
Chicago/Turabian StyleRzymski, Piotr, Monika Pazgan-Simon, Juliusz Kamerys, Anna Moniuszko-Malinowska, Katarzyna Sikorska, Joanna Wernik, Dorota Zarębska-Michaluk, Łukasz Supronowicz, Barbara Sobala-Szczygieł, Agata Skrzat-Klapaczyńska, and et al. 2022. "Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland" Vaccines 10, no. 4: 557. https://doi.org/10.3390/vaccines10040557
APA StyleRzymski, P., Pazgan-Simon, M., Kamerys, J., Moniuszko-Malinowska, A., Sikorska, K., Wernik, J., Zarębska-Michaluk, D., Supronowicz, Ł., Sobala-Szczygieł, B., Skrzat-Klapaczyńska, A., Simon, K., Piekarska, A., Czupryna, P., Pawłowska, M., Brzdęk, M., Jaroszewicz, J., Kowalska, J., Renke, M., & Flisiak, R. (2022). Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines, 10(4), 557. https://doi.org/10.3390/vaccines10040557











