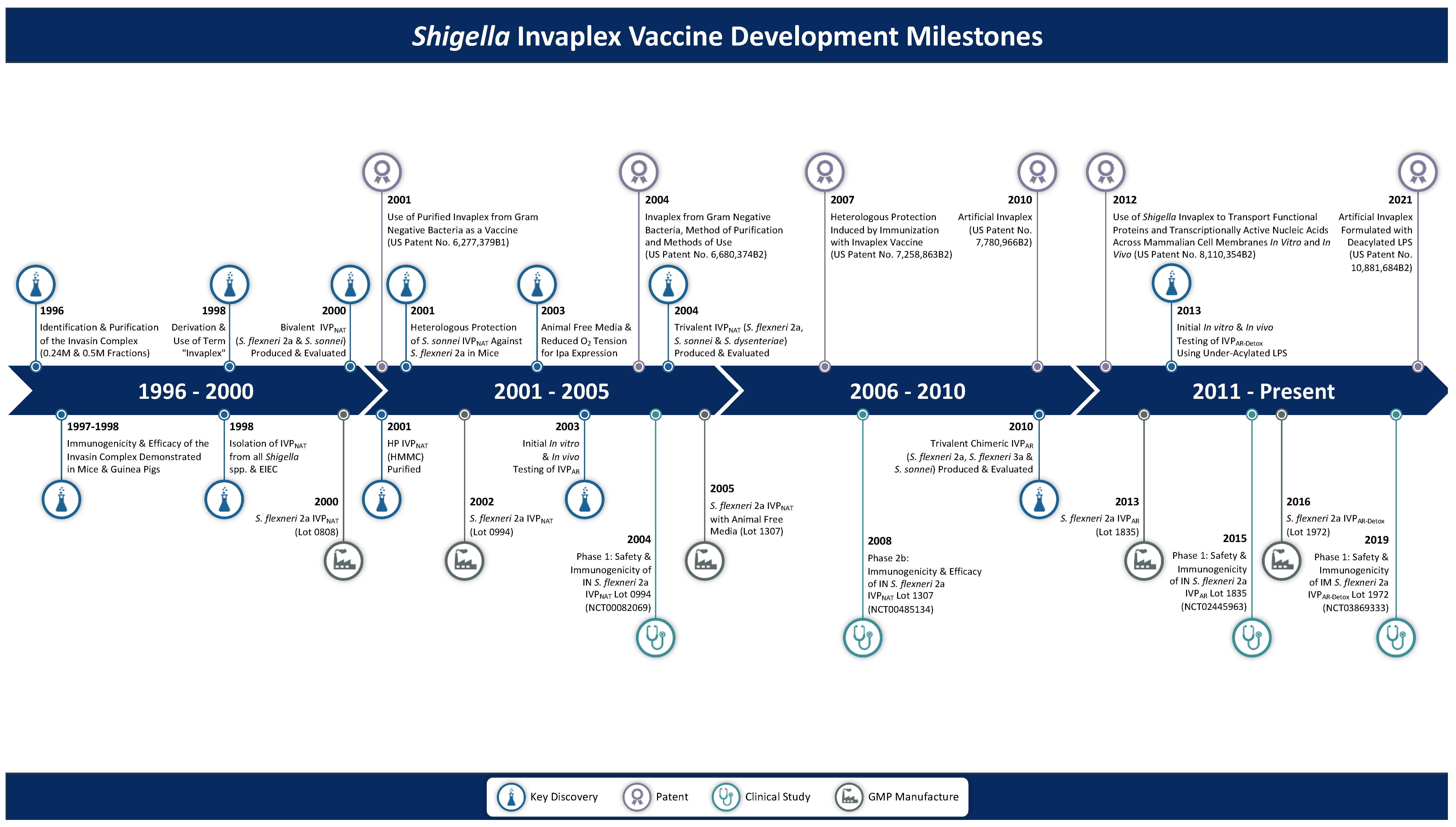
From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution
Abstract
1. Introduction
2. Shigella Antigens
3. Native Invaplex (InvaplexNAT)
3.1. Discovery of Invaplex
3.2. Biological Properties of InvaplexNAT
3.3. Intranasal Delivery of Invaplex without an Adjuvant
3.4. Immunogenicity of InvaplexNAT: Assay Development and Results
3.5. Heterologous Immunity Induced by Invaplex
3.6. cGMP Manufacture of InvaplexNAT
3.7. Clinical Evaluation of S. flexneri 2a InvaplexNAT
| Study Title | Phase | ClinicalTrials.gov Identifier | Study Start Date | Product (cGMP Lot No.) | Reference |
|---|---|---|---|---|---|
| Invaplex 50 Vaccine Dose-Ranging | 1 | NCT00082069 | April 2004 | S. flexneri 2a InvaplexNAT (0994) | [42] |
| Shigella flexneri 2a Invaplex 50 Vaccine Dose Finding and Assessment of Protection | 1 | NCT00485134 | August 2007 | S. flexneri 2a InvaplexNAT (0994 and 1307) | [7] |
| 2b 1 | January 2008 | S. flexneri 2a InvaplexNAT (1307) | [60] | ||
| 2b | November 2008 | S. flexneri 2a InvaplexNAT (1307) | |||
| Safety and Immunogenicity of Artificial Invaplex (Shigella flexneri 2a InvaplexAR) Administered Intranasally to Healthy, Adult Volunteers | 1 | NCT02445963 | October 2015 | S. flexneri 2a InvaplexAR (1835) | [63] |
| A Phase 1 Double-blind, Placebo-controlled, Dose Escalating Study of Intramuscular Detoxified Shigella flexneri 2a Artificial Invasin Complex (InvaplexAR-Detox) Vaccine | 1 | NCT03869333 | March 2019 | S. flexneri 2a InvaplexAR-Detox (1972) | [64] |
3.8. Transition to an Invaplex with Greater Effectiveness
4. Artificial Invaplex (InvaplexAR)
4.1. Development of InvaplexAR
4.2. cGMP Manufacture of InvaplexAR
4.3. Clinical Evaluation of S. flexneri 2a InvaplexAR
5. Artificial Detoxified Invaplex (InvaplexAR-Detox)
5.1. Development and Pre-Clinical Testing of InvaplexAR-Detox
5.2. Pre-Clinical Studies with InvaplexAR-Detox
5.3. Clinical Evaluation of InvaplexAR-Detox
6. Next Steps and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Copyright Statement
References
- Ferreccio, C.; Prado, V.; Ojeda, A.; Cayyazo, M.; Abrego, P.; Guers, L.; Levine, M.M. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am. J. Epidemiol. 1991, 134, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Formal, S.B.; Oaks, E.V.; Olsen, R.E.; Wingfield-Eggleston, M.; Snoy, P.J.; Cogan, J.P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 1991, 164, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Taylor, D.N.; Trofa, A.C.; Sadoff, J.; Chu, C.; Bryla, D.; Shiloach, J.; Cohen, D.; Ashkenazi, S.; Lerman, Y.; Egan, W.; et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect. Immun. 1993, 61, 3678–3687. [Google Scholar] [CrossRef]
- Farzam, N.; Ramon-Saraf, R.; Banet-Levi, Y.; Lerner-Geva, L.; Ashkenazi, S.; Kubler-Kielb, J.; Vinogradov, E.; Robbins, J.B.; Schneerson, R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 2017, 35, 4990–4996. [Google Scholar] [CrossRef]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef]
- Oaks, E.V.; Hale, T.L.; Formal, S.B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect. Immun. 1986, 53, 57–63. [Google Scholar] [CrossRef]
- Coster, T.S.; Hoge, C.W.; VanDeVerg, L.L.; Hartman, A.B.; Oaks, E.V.; Venkatesan, M.M.; Cohen, D.; Robin, G.; Fontaine-Thompson, A.; Sansonetti, P.J.; et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 1999, 67, 3437–3443. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Taylor, D.N.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; Nataro, J.P.; Venkatesan, M.; Hartman, A.; Picking, W.D.; Katz, D.E.; et al. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 2002, 70, 2016–2021. [Google Scholar] [CrossRef][Green Version]
- Kotloff, K.L.; Noriega, F.; Losonsky, G.A.; Sztein, M.B.; Wasserman, S.S.; Nataro, J.P.; Levine, M.M. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 1996, 64, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Oaks, E.; Kaminski, R. Use of Shigella Invaplex to Transport Functional Proteins and Transcriptionally Active Nucleic Acids Across Mammalian Cell Membranes In Vitro and In Vivo. U.S. Patent 7,632,659, 15 December 2009. [Google Scholar]
- Oaks, E.; Kaminski, R. Use of Shigella Invaplex to Transport Functional Proteins and Transcriptionally Active Nucleic Acids Across Mammalian Cell Membranes In Vitro and In Vivo. U.S. Patent 8,110,354, 7 February 2012. [Google Scholar]
- Van de Verg, L.L.; Herrington, D.A.; Boslego, J.; Lindberg, A.A.; Levine, M.M. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J. Infect. Dis. 1992, 166, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Wilson, R.L.; Singletary, M.; Blanchard, J.L.; Aldovini, A.; Kaminski, R.W.; Oaks, E.V.; Kozlowski, P.A. Invaplex functions as an intranasal adjuvant for subunit and DNA vaccines co-delivered in the nasal cavity of nonhuman primates. Vaccine X 2021, 8, 100105. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.A.; Buysse, J.M.; Oaks, E.V. Shigella flexneri invasion plasmid antigens B and C: Epitope location and characterization with monoclonal antibodies. Infect. Immun. 1988, 56, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Sansonetti, P.; Parsot, C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994, 13, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Prevost, M.C.; Gounon, P.; Sansonetti, P.; Dehio, C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 1996, 93, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Tobe, T.; Yoshikawa, M.; Sasakawa, C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995, 14, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Rong, Z.C.; Ekwall, E.; Forsum, U.; Lindberg, A.A. Serum antibody responses against shigella lipopolysaccharides and invasion plasmid-coded antigens in shigella infected Swedish patients. Scand. J. Infect. Dis. 1993, 25, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Samandari, T.; Kotloff, K.L.; Losonsky, G.A.; Picking, W.D.; Sansonetti, P.J.; Levine, M.M.; Sztein, M.B. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J. Immunol. 2000, 164, 2221–2232. [Google Scholar] [CrossRef]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Hochberg, A.; Ashkenazi, S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccines Immunother. 2019, 15, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Turbyfill, K.R.; Oaks, E.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 1996.
- Turbyfill, K.R.; Hartman, A.B.; Oaks, E.V. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect. Immun. 2000, 68, 6624–6632. [Google Scholar] [CrossRef] [PubMed]
- Oaks, E.; Turbyfill, K.R.; Hartman, A. Use of Purified Invaplex from Gram Negative Bacteria as a Vaccine. U.S. Patent 6,277,379, 21 August 2001. [Google Scholar]
- Oaks, E.V.; Turbyfill, K.R. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine 2006, 24, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Oaks, E.; Turbyfill, K.R.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 2011.
- Menard, R.; Sansonetti, P.J.; Parsot, C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1993, 175, 5899–5906. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.W.; Turbyfill, K.R.; Oaks, E.V. Mucosal adjuvant properties of the Shigella invasin complex. Infect. Immun. 2006, 74, 2856–2866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaminski, R.W.; Turbyfill, K.R.; Chao, C.; Ching, W.M.; Oaks, E.V. Mucosal adjuvanticity of a Shigella invasin complex with dna-based vaccines. Clin. Vaccine Immunol. 2009, 16, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Oaks, E.; Turbyfill, K.R. Invaplex from Gram Negative Bacteria, Method of Purification and Methods of Use. U.S. Patent 6,245,892, 12 June 2001. [Google Scholar]
- Oaks, E.; Turbyfill, K.R. Invaplex from Gram Negative Bacteria, Method of Purification and Methods of Use. U.S. Patent 6,680,374, 20 January 2004. [Google Scholar]
- Clarkson, K.A.; Frenck, R.W., Jr.; Dickey, M.; Suvarnapunya, A.E.; Chandrasekaran, L.; Weerts, H.P.; Heaney, C.D.; McNeal, M.; Detizio, K.; Parker, S.; et al. Immune Response Characterization after Controlled Infection with Lyophilized Shigella sonnei 53G. mSphere 2020, 5, e00988-19. [Google Scholar] [CrossRef] [PubMed]
- Ndungo, E.; Randall, A.; Hazen, T.H.; Kania, D.A.; Trappl-Kimmons, K.; Liang, X.; Barry, E.M.; Kotloff, K.L.; Chakraborty, S.; Mani, S.; et al. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere 2018, 3, e00260-18. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; DiLorenzo, S.C.; Walker, R.I. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 2001, 69, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Russell, M.W.; Wu, H.Y.; Huang, W.Q.; Compans, R.W.; Mestecky, J. Compartmentalization within the common mucosal immune system. Adv. Exp. Med. Biol. 1995, 371A, 97–101. [Google Scholar] [CrossRef]
- Kozlowski, P.A.; Williams, S.B.; Lynch, R.M.; Flanigan, T.P.; Patterson, R.R.; Cu-Uvin, S.; Neutra, M.R. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: Influence of the menstrual cycle. J. Immunol. 2002, 169, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.F.; Montemarano, A.D.; Mallett, C.P.; Taylor, D.N.; Hale, T.L.; Lowell, G.H. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect. Immun. 2001, 69, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Mulczyk, M.; Witkowska, D.; Romanowska, E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect. Immun. 1980, 30, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Mallett, C.P.; Hale, T.L.; Kaminski, R.W.; Larsen, T.; Orr, N.; Cohen, D.; Lowell, G.H. Intransal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect. Immun. 1995, 63, 2382–2386. [Google Scholar] [CrossRef]
- Tribble, D.; Kaminski, R.; Cantrell, J.; Nelson, M.; Porter, C.; Baqar, S.; Williams, C.; Arora, R.; Saunders, J.; Ananthakrishnan, M.; et al. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine 2010, 28, 6076–6085. [Google Scholar] [CrossRef]
- Riddle, M.S.; Kaminski, R.W.; Williams, C.; Porter, C.; Baqar, S.; Kordis, A.; Gilliland, T.; Lapa, J.; Coughlin, M.; Soltis, C.; et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 2011, 29, 7009–7019. [Google Scholar] [CrossRef]
- Weerts, H.P.; Yu, J.; Kaminski, R.W.; Nahm, M.H. A High-throughput Shigella-specific Bactericidal Assay. J. Vis. Exp. 2019, 144, e59164. [Google Scholar] [CrossRef] [PubMed]
- Nahm, M.H.; Yu, J.; Weerts, H.P.; Wenzel, H.; Tamilselvi, C.S.; Chandrasekaran, L.; Pasetti, M.F.; Mani, S.; Kaminski, R.W. Development, Interlaboratory Evaluations, and Application of a Simple, High-Throughput Shigella Serum Bactericidal Assay. mSphere 2018, 3, e00146-18. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Molesti, E.; Saul, A.; Giannelli, C.; Micoli, F.; Necchi, F. Intra-Laboratory Evaluation of Luminescence Based High-Throughput Serum Bactericidal Assay (L-SBA) to Determine Bactericidal Activity of Human Sera against Shigella. High Throughput 2020, 9, 14. [Google Scholar] [CrossRef]
- Oaks, E.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 2004.
- Frenck, R.W., Jr.; Baqar, S.; Alexander, W.; Dickey, M.; McNeal, M.; El-Khorazaty, J.; Baughman, H.; Hoeper, A.; Barnoy, S.; Suvarnapunya, A.E.; et al. A Phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine 2018, 36, 4880–4889. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Kaminski, R.W.; Oaks, E.V. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine 2008, 26, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Palchaudhuri, S. Nucleotide sequence of the ipaBCD structural genes of Shigella dysenteriae. Mol. Microbiol. 1991, 5, 2217–2221. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.; Islam, D.; Odundo, E.; Kaminski, R.; Hang, J.; Ruamsap, N.; Sakpaisal, P.; Silapong, S.; Oransathid, W.; Lertsethtakarn, P.; et al. Shigella IpaB/C/D Invasion Plasmid Sequence Conservation in a Wide Range of Clinical Isolates. In Proceedings of the Military Health System Research Symposium, Fort Lauderdale, FL, USA, 18 August 2014. [Google Scholar]
- Oaks, E.; Turbyfill, K.R. Heterologous Protection Induced by Immunization with Invaplex Vaccine. U.S. Patent 7,258,863, 21 August 2007. [Google Scholar]
- Turbyfill, K.R.; Clarkson, K.A.; Vortherms, A.R.; Oaks, E.V.; Kaminski, R.W. Assembly, Biochemical Characterization, Immunogenicity, Adjuvanticity, and Efficacy of Shigella Artificial Invaplex. mSphere 2018, 3, e00583-17. [Google Scholar] [CrossRef] [PubMed]
- Chitradevi, S.T.; Kaur, G.; Uppalapati, S.; Yadav, A.; Singh, D.; Bansal, A. Co-administration of rIpaB domain of Shigella with rGroEL of S. Typhi enhances the immune responses and protective efficacy against Shigella infection. Cell. Mol. Immunol. 2015, 12, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Becerra, F.J.; Chen, X.; Dickenson, N.E.; Choudhari, S.P.; Harrison, K.; Clements, J.D.; Picking, W.D.; Van De Verg, L.L.; Walker, R.I.; Picking, W.L. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect. Immun. 2013, 81, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Becerra, F.J.; Kissmann, J.M.; Diaz-McNair, J.; Choudhari, S.P.; Quick, A.M.; Mellado-Sanchez, G.; Clements, J.D.; Pasetti, M.F.; Picking, W.L. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 2012, 80, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 2018.
- Turbyfill, K.R.; Oaks, E.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 2001.
- Turbyfill, K.R.; Karwoski, J.; Pradier, A.; Ash, L.; Kaminski, R.; Oaks, E. Fermentation conditions for production of large quantities of the subcellular invasin complex (Invaplex) vaccine from Shigella flexneri. In Proceedings of the American Society for Microbiology, Annual Meeting, Orlando, FL, USA, 21–25 May 2006. [Google Scholar]
- Harro, C.; Riddle, M.S.; Kaminski, R.; Turbyfill, K.R.; Gormley, R.; Porter, C.; Ranallo, R.T.; Kordis, A.; Buck, M.; Jones, A.; et al. Shigella flexneri 2a Invaplex 50 intranasal vaccine phase 2b challenge study. In Proceedings of the Vaccines for Enteric Diseases (VED), Malaga, Spain, 9–11 September 2009. [Google Scholar]
- Turbyfill, K.R.; Oaks, E.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Unpublished Observations. 2006.
- Hartman, A.B.; Powell, C.J.; Schultz, C.L.; Oaks, E.V.; Eckels, K.H. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect. Immun. 1991, 59, 4075–4083. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, K.A.; Duplessis, C.; Turbyfill, K.R.; Porter, C.; Gutierrez, R.L.; Riddle, M.S.; Lee, T.; Weerts, H.; Sumlin, S.C.; Lynen, A.; et al. A Phase 1 Open-label, Dose Escalating Study of Shigella flexneri 2a Artificial Invaplex administered intranasally to healthy, adult volunteers. In Proceedings of the Vaccines for Enteric Diseases (VED), Albufeira, Portugal, 9–11 October 2017. [Google Scholar]
- Clarkson, K.A.; Gutierrez, R.L.; Turbyfill, K.R.; Vortherms, A.R.; Lynen, A.; Barnard, B.A.; Weerts, H.; Porter, C.; Maier, N.; Erdem, R.; et al. Clinical Evaluation of Shigella flexneri 2a Detoxified Artificial Invaplex. In Proceedings of the Vaccines Against Shigella and ETEC (VASE), Virtual Symposium, 28–30 September 2021. [Google Scholar]
- Westphal, O.; Jann, K. Bacterial Lipopolysaccharides: Extraction with Phenol-Water and Further Applications of the Procedure; Academic Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Picking, W.L.; Mertz, J.A.; Marquart, M.E.; Picking, W.D. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr. Purif. 1996, 8, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Birket, S.E.; Harrington, A.T.; Espina, M.; Smith, N.D.; Terry, C.M.; Darboe, N.; Markham, A.P.; Middaugh, C.R.; Picking, W.L.; Picking, W.D. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 2007, 46, 8128–8137. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Sansonetti, P.; Parsot, C.; Vasselon, T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 1994, 79, 515–525. [Google Scholar] [CrossRef]
- Dickenson, N.E.; Choudhari, S.P.; Adam, P.R.; Kramer, R.M.; Joshi, S.B.; Middaugh, C.R.; Picking, W.L.; Picking, W.D. Oligomeric states of the Shigella translocator protein IpaB provide structural insights into formation of the type III secretion translocon. Protein Sci. 2013, 22, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Hume, P.J.; McGhie, E.J.; Hayward, R.D.; Koronakis, V. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol. Microbiol. 2003, 49, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Mounier, J.; Boncompain, G.; Senerovic, L.; Lagache, T.; Chretien, F.; Perez, F.; Kolbe, M.; Olivo-Marin, J.C.; Sansonetti, P.J.; Sauvonnet, N. Shigella effector IpaB-induced cholesterol relocation disrupts the Golgi complex and recycling network to inhibit host cell secretion. Cell Host Microbe 2012, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Senerovic, L.; Tsunoda, S.P.; Goosmann, C.; Brinkmann, V.; Zychlinsky, A.; Meissner, F.; Kolbe, M. Spontaneous formation of IpaB ion channels in host cell membranes reveals how Shigella induces pyroptosis in macrophages. Cell Death Dis. 2012, 3, e384. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Turbyfill, K.R.; Clarkson, K.A.; Vortherms, A.R.; Oaks, E.; Kaminski, R.; (Walter Reed Army Institute of Research, Silver Spring, MD, USA). Manuscript in Preparation. 2022.
- Clarkson, K.A.; Duplessis, C.; Turbyfill, K.R.; Porter, C.; Weerts, H.; Gutierrez, R.L.; Riddle, M.S.; Lynen, A.; Oaks, E.; Kaminski, R.; Walter Reed Army Institute of Research, Silver Spring, MD, USA. A Phase 1 Open-label, Dose Escalating Study of Shigella flexneri 2a Artificial Invaplex (InvaplexAR) Administered Intranasally to Healthy, Adult Volunteers. 2022; Manuscript in Preparation. [Google Scholar]
- D’Hauteville, H.; Khan, S.; Maskell, D.J.; Kussak, A.; Weintraub, A.; Mathison, J.; Ulevitch, R.J.; Wuscher, N.; Parsot, C.; Sansonetti, P.J. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 2002, 168, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, R.T.; Kaminski, R.W.; George, T.; Kordis, A.A.; Chen, Q.; Szabo, K.; Venkatesan, M.M. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect. Immun. 2010, 78, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, K.A.; Gutierrez, R.L.; Turbyfill, K.R.; Detizio, K.; Vortherms, A.R.; Lynen, A.; Barnard, B.A.; Weerts, H.; Porter, C.; Maier, N.; et al.; Walter Reed Army Institute of Research, Silver Spring, MD, USA GMP manufacture, characterization and clinical evaluation of Shigella flexneri 2a detoxified artificial invaplex. 2022; Manuscript in Preparation. [Google Scholar]
- Shim, D.H.; Suzuki, T.; Chang, S.Y.; Park, S.M.; Sansonetti, P.J.; Sasakawa, C.; Kweon, M.N. New animal model of shigellosis in the Guinea pig: Its usefulness for protective efficacy studies. J. Immunol. 2007, 178, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, R.L.; Bolick, D.T.; Swann, J.R. Modeling Enteropathy or Diarrhea with the Top Bacterial and Protozoal Pathogens: Differential Determinants of Outcomes. ACS Infect. Dis. 2021, 7, 1020–1031. [Google Scholar] [CrossRef]
- Clarkson, K.A.; Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.; Porter, C.K.; Chakraborty, S.; Brubaker, J.; Elwood, D.; et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine 2021, 66, 103308. [Google Scholar] [CrossRef] [PubMed]
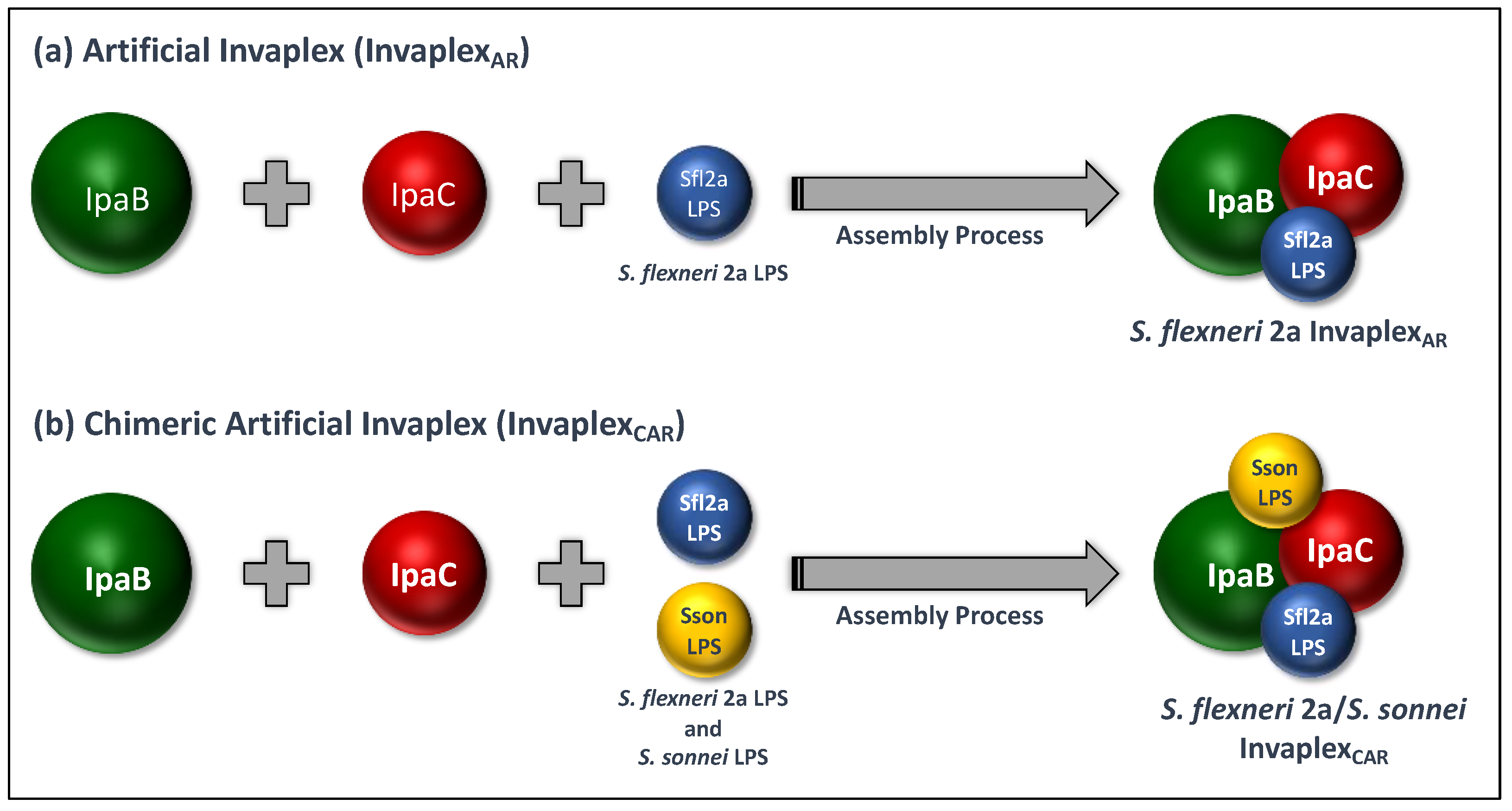
| Attribute/Property | 1st Generation | 2nd Generation | 3rd Generation |
|---|---|---|---|
| Name | Native Invaplex (InvaplexNAT) | Artificial Invaplex (InvaplexAR) | Detoxified Artificial Invaplex (InvaplexAR-Detox) |
| Date of Discovery | 1996 | 2003 | 2013 |
| Source | Isolated from wild-type shigellae | Assembled from recombinant proteins and wild-type LPS | Assembled from recombinant proteins and under-acylated LPS |
| Complex Composition |
|
|
|
| First cGMP Manufacture Date | 2000 | 2013 | 2016 |
| Adjuvant Activity | ✓ | ✓ | ✓ |
| Induces Uptake (Non-Phagocytic Cells) | ✓ | ✓ | ✓ |
| Produced for Multiple Shigella Serotypes | S. flexneri 2a S. sonnei S. dysenteriae S. boydii | S. flexneri 2a S. flexneri 3a S. flexneri 6 S. flexneri 1b S. dysenteriae S. sonnei | S. flexneri 2a S. sonnei |
| Route of Immunization | Intranasal | Intranasal | Parenteral |
| Efficacious in Mice | ✓ | ✓ | ✓ |
| Efficacious in Guinea Pigs | ✓ | ✓ | ✓ |
| Heterologous Protection (Mice) | ✓ | ✓ | Not Tested |
| Analysis | InvaplexNAT (cGMP Lot 1307) | InvaplexAR (cGMP Lot 1835) | InvaplexAR-Detox (cGMP Lot 1972) | |
|---|---|---|---|---|
| Endotoxin (EU/mL) 1 | 0.6 × 107 | 5.3 × 107 | 4.6 × 107 | |
| Protein Concentration (mg/mL) 2 | 3.5 | 2.9 | 2.8 | |
| IpaC: IpaB Ratio 3 | 1.5:1 | 6.8:1 | 5.4:1 | |
| LPS: Total Protein Ratio (EU/mg) | 0.17 × 107 | 1.8 × 107 | 1.6 × 107 | |
| SEC Retention Time (mins) 4 | 17.4 Multiple Peaks | 15.7 Single Peak | 16.2 Single Peak | |
| Size, DH (nm ± standard deviation) 5 | 10 ± 3 | 24 ± 3 | 24 ± 4 | |
| Immunogenicity 6 | 2036 (100%) p < 0.001 | 8146 (100%) p < 0.0001 | 146,295 (100%) p < 0.0001 | |
| Protective Efficacy 7 | 83% p < 0.001 | 100% p < 0.0001 | 75% p = 0.0003 | |
| GLP Toxicology | No significant histopathology in mice after IN immunization | No significant histopathology in mice after IN immunization | No significant adverse findings in rabbits after IM immunization | |
| Pyrogenicity | Negative in rabbits (IN immunization) | Negative in rabbits (IN immunization) | Monocyte Activation Test—PASS | |
| Stability at −80 °C (no loss of antigen content or immunogenicity) | ≥3 years | ≥5 years | ≥5 years | |
| Stage of Clinical Development | Phase 1: Safe and immunogenic P2b: Less immunogenic and not protective | Phase 1: Safe and immunogenic | Phase 1: Safe and highly immunogenic | |
| Lowest Immunogenic Dose for ≥50% Seroconversion in Humans | Anti-InvaplexNAT IgG | 690 µg | 50 µg | 2.5 µg |
| Anti-LPS IgG | 690 µg | 250 µg | 2.5 µg | |
| Shigella Species | Endotoxin (×106 EU/mL) 1 | IpaC:IpaB Ratio 2 | SEC-HPLC Retention Time (min) 3 | DLS DH (nm) 4 | Immunogenicity (Responses Directed to IpaB, IpaC and LPS) 5 | Efficacy (p Value) 6 |
|---|---|---|---|---|---|---|
| S. flexneri 2a | 63 | 7.8:1 | 15.6 | 19.5 ± 7.7 | Yes | 75% (p = 0.0005) |
| S. flexneri 3a | 16 | 3.8:1 | 16.2 | 19.5 ± 4.8 | Yes | 70% (p = 0.012) |
| S. flexneri 6 | 220 | 3.9:1 | 16.0 | 28.2 ± 0.4 | Yes | 71% (p = 0.0213) |
| S. sonnei | 5.7 | 2.6:1 | 16.1 | 19.5 ± 4.0 | Yes | 92% (p < 0.0001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turbyfill, K.R.; Clarkson, K.A.; Oaks, E.V.; Kaminski, R.W. From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines 2022, 10, 548. https://doi.org/10.3390/vaccines10040548
Turbyfill KR, Clarkson KA, Oaks EV, Kaminski RW. From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines. 2022; 10(4):548. https://doi.org/10.3390/vaccines10040548
Chicago/Turabian StyleTurbyfill, K. Ross, Kristen A. Clarkson, Edwin V. Oaks, and Robert W. Kaminski. 2022. "From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution" Vaccines 10, no. 4: 548. https://doi.org/10.3390/vaccines10040548
APA StyleTurbyfill, K. R., Clarkson, K. A., Oaks, E. V., & Kaminski, R. W. (2022). From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines, 10(4), 548. https://doi.org/10.3390/vaccines10040548






