Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19
Abstract
:1. Introduction
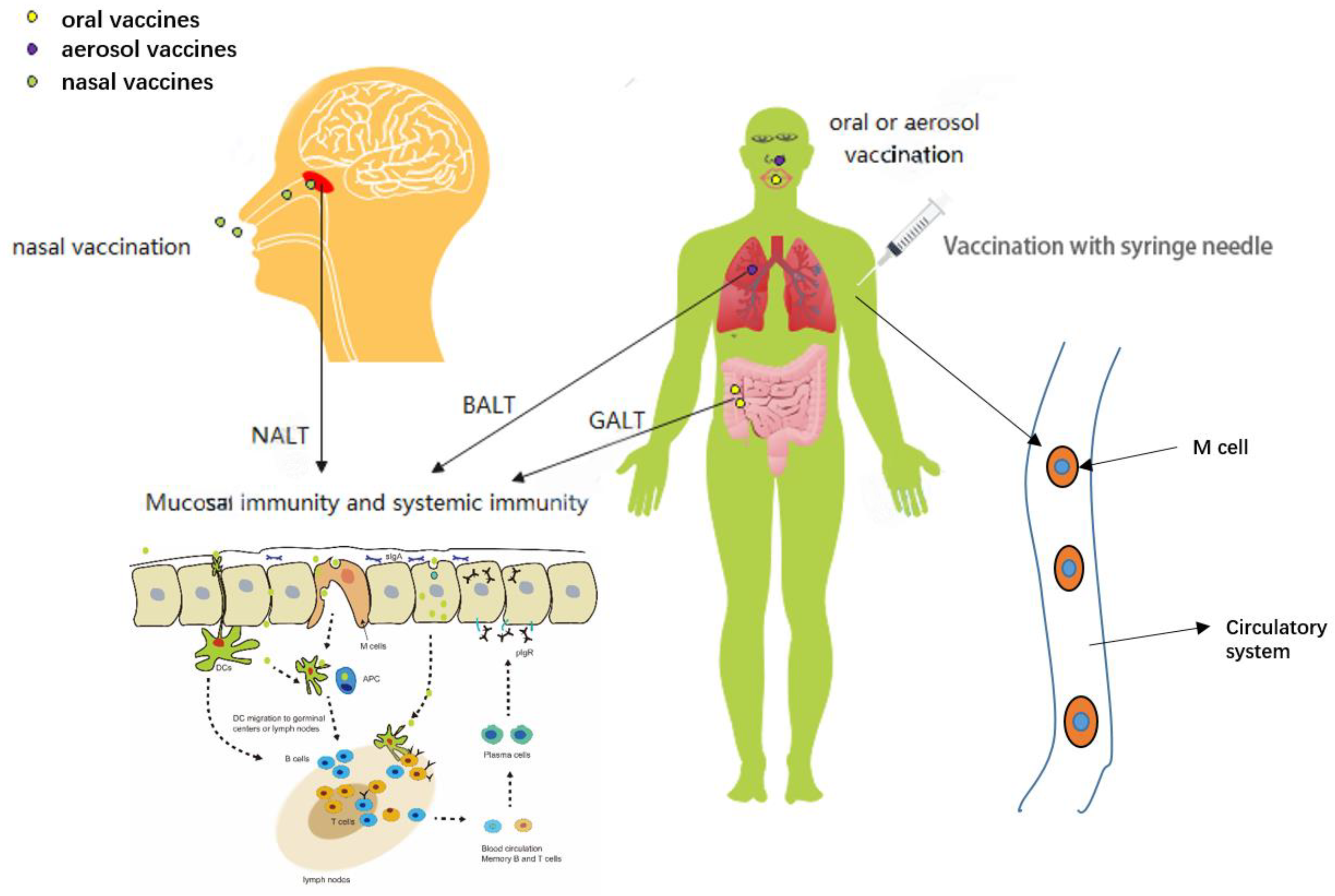
2. The Route of Vaccination
2.1. Vaccination with Syringe Needle
2.2. Mucosal Vaccination
3. The Current Status of Nasal Vaccines in SARS-CoV-2
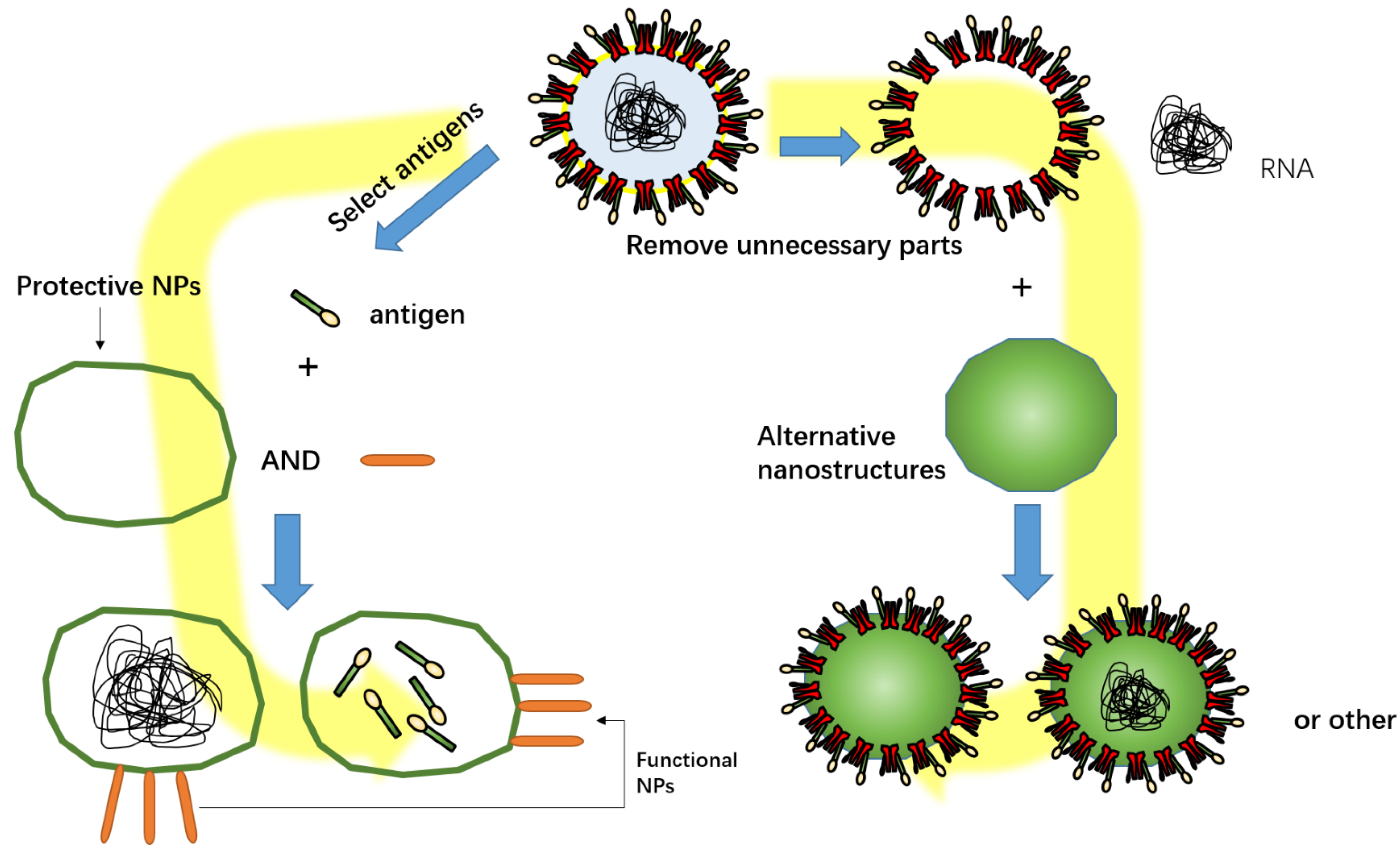
4. Nanovaccines
4.1. Polysaccharide Nanoparticles
4.2. Lipid Nanoparticles
4.2.1. Liposomes
4.2.2. Other Lipid Nanoparticles
4.3. Protein Nanoparticles
Self-Assembled Proteins
4.4. Nano-Biomimetic Delivery Vehicles
4.5. Polymer Nanoparticles
5. Combination of Antigen and NPs
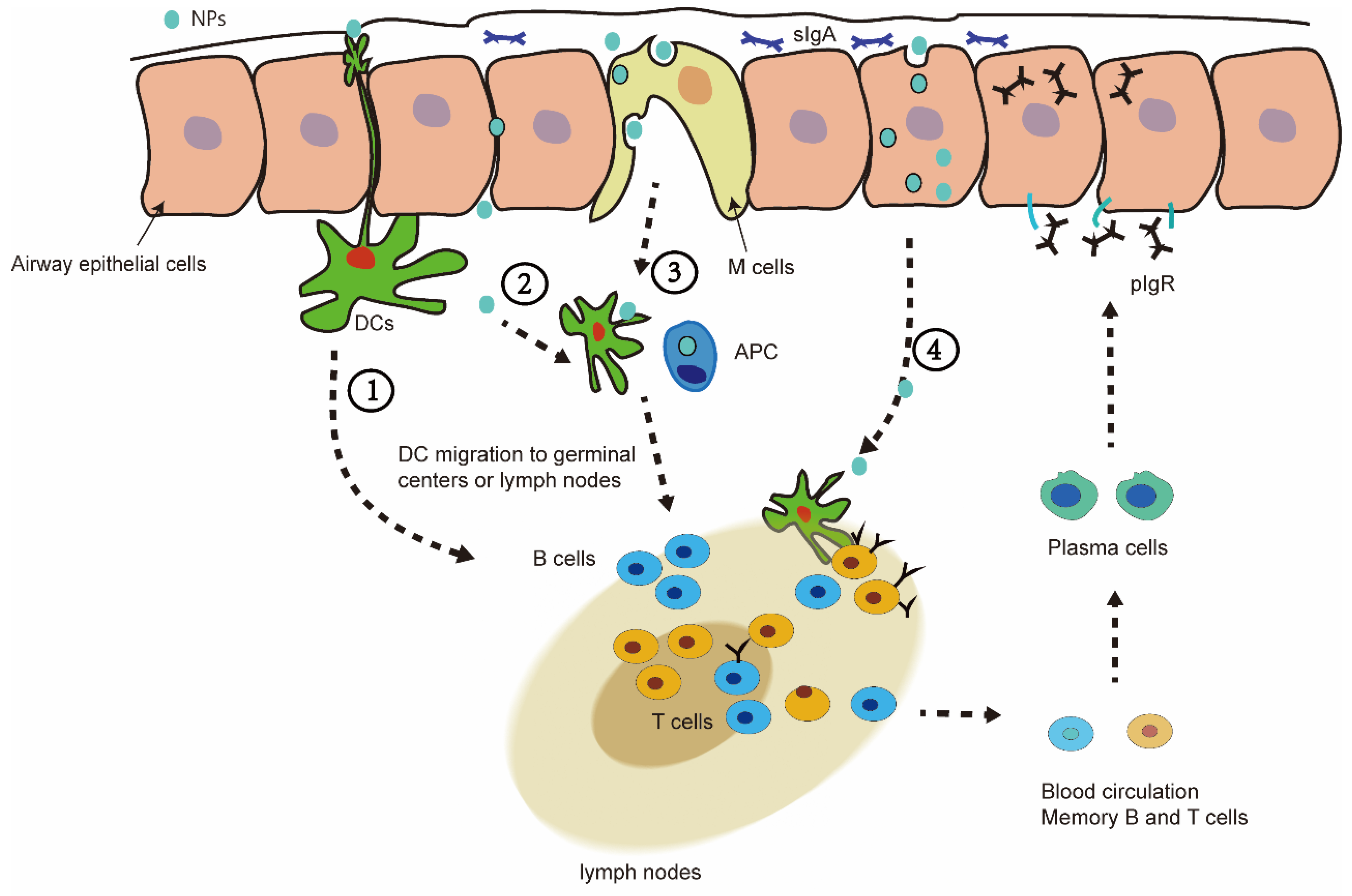
6. Nasal Mucosal Immunity of Nanovaccine
7. Why Choose Nasal Nanovaccine in SARS-CoV-2?
7.1. Some People Cannot Effectively Resist SARS-CoV-2 after Vaccination
7.2. Troubling Feature: Mutation
8. Future and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 26 November 2021).
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Kaka, A.S.; MacDonald, R.; Greer, N.; Obley, A.; Duan-Porter, W. Remdesivir for Adults With COVID-19: A Living Systematic Review for American College of Physicians Practice Points. Ann. Intern. Med. 2021, 174, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Bistrovic, P.; Lucijanic, M. Remdesivir might induce changes in electrocardiogram beyond bradycardia in patients with Coronavirus disease 2019 (COVID-19)—The pilot study. J. Med. Virol. 2021, 93, 5724. [Google Scholar] [CrossRef]
- Attena, E.; Albani, S.; Maraolo, A.E.; Mollica, M.; De Rosa, A.; Pisapia, R.; Fiorentino, G.; Parrella, R.; Severino, S.; Russo, V. Remdesivir-Induced Bradycardia in COVID-19: A Single Center Prospective Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e009811. [Google Scholar] [CrossRef]
- Chow, E.J.; Maust, B.; Kazmier, K.M.; Stokes, C. Sinus Bradycardia in a Pediatric Patient Treated With Remdesivir for Acute Coronavirus Disease 2019: A Case Report and a Review of the Literature. J. Pediatr. Infect. Dis. Soc. 2021, 10, 926–929. [Google Scholar] [CrossRef]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Gaio, M.; Zinzi, A.; Scavone, C.; Gargano, F.; Coscioni, E.; Rossi, F.; Capuano, A. Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2021, 14, 611. [Google Scholar] [CrossRef]
- Selvaraj, V.; Bavishi, C.; Patel, S.; Dapaah-Afriyie, K. Complete heart block associated with Remdesivir in COVID-19: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab200. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, H.; Ari, M.M.; Dashtbin, S.; Fathollahi, M.; Hossainpour, H.; Alvandi, A.; Moradi, J.; Abiri, R. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int. Immunopharmacol. 2021, 96, 107763. [Google Scholar] [CrossRef] [PubMed]
- Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (Recovery): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; Prudon, B.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef]
- Palamenghi, L.; Barello, S.; Boccia, S.; Graffigna, G. Mistrust in biomedical research and vaccine hesitancy: The forefront challenge in the battle against COVID-19 in Italy. Eur. J. Epidemiol. 2020, 35, 785–788. [Google Scholar] [CrossRef]
- Peretti-Watel, P.; Seror, V.; Cortaredona, S.; Launay, O.; Raude, J.; Verger, P.; Fressard, L.; Beck, F.; Legleye, S.; l’Haridon, O.; et al. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet. Infect. Dis. 2020, 20, 769–770. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 26 November 2021).
- McLenon, J.; Rogers, M.A.M. The fear of needles: A systematic review and meta-analysis. J. Adv. Nurs. 2019, 75, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modanloo, S.; Stacey, D.; Dunn, S.; Choueiry, J.; Harrison, D. Parent resources for early childhood vaccination: An online environmental scan. Vaccine 2019, 37, 7493–7500. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Connor, R.I.; Brickley, E.B.; Wieland-Alter, W.F.; Ackerman, M.E.; Weiner, J.A.; Modlin, J.F.; Bandyopadhyay, A.S.; Wright, P.F. Mucosal immunity to poliovirus. Mucosal Immunol. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- McCright, J.; Ramirez, A.; Amosu, M.; Sinha, A.; Bogseth, A.; Maisel, K. Targeting the Gut Mucosal Immune System Using Nanomaterials. Pharmaceutics 2021, 13, 1755. [Google Scholar] [CrossRef]
- Nechaeva, E. Development of oral microencapsulated forms for delivering viral vaccines. Expert Rev. Vaccines 2002, 1, 385–397. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef]
- LiCalsi, C.; Maniaci, M.J.; Christensen, T.; Phillips, E.; Ward, G.H.; Witham, C. A powder formulation of measles vaccine for aerosol delivery. Vaccine 2001, 19, 2629–2636. [Google Scholar] [CrossRef]
- Lu, D.; Hickey, A.J. Pulmonary vaccine delivery. Expert Rev. Vaccines 2007, 6, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.X.; Wei, Y.N.; Li, G.L.; Lu, X.M.; Wan, X.F.; Pharr, G.T.; Wang, Z.W.; Kong, M.; Gan, Y.; Bai, F.F.; et al. Development and validation of an attenuated Mycoplasma hyopneumoniae aerosol vaccine. Vet. Microbiol. 2013, 167, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, R.; Nehul, S.; Tomar, S. Prospects for mucosal vaccine: Shutting the door on SARS-CoV-2. Hum. Vaccines Immunother. 2020, 16, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021, 14, 305–316. [Google Scholar] [CrossRef]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H., III; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Chan, R.W.Y.; Liu, S.; Cheung, J.Y.; Tsun, J.G.S.; Chan, K.C.; Chan, K.Y.Y.; Fung, G.P.G.; Li, A.M.; Lam, H.S. The Mucosal and Serological Immune Responses to the Novel Coronavirus (SARS-CoV-2) Vaccines. Front. Immunol. 2021, 12, 744887. [Google Scholar] [CrossRef]
- Neidleman, J.; Luo, X.; McGregor, M.; Xie, G.; Murray, V.; Greene, W.C.; Lee, S.A.; Roan, N.R. mRNA vaccine-induced T cells respond identically to SARS-CoV-2 variants of concern but differ in longevity and homing properties depending on prior infection status. eLife 2021, 10, e72619. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Tscherne, A.; Schwarz, J.H.; Rohde, C.; Kupke, A.; Kalodimou, G.; Limpinsel, L.; Okba, N.M.A.; Bošnjak, B.; Sandrock, I.; Odak, I.; et al. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2026207118. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, Y.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat. Commun. 2020, 11, 6121. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Garaigorta, U.; Pérez, P.; Lázaro-Frías, A.; Zamora, C.; Gastaminza, P.; Del Fresno, C.; Casasnovas, J.M.; Sorzano CÓ, S.; Sancho, D.; et al. COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice. J. Virol. 2021, 95, e02260-20. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol. Appl. Pharmacol. 2016, 299, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Bernocchi, B.; Carpentier, R.; Betbeder, D. Nasal nanovaccines. Int. J. Pharm. 2017, 530, 128–138. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the dots in drug delivery: A tour d’horizon of chitosan-based nanocarriers system. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef] [PubMed]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Gao, X.; Gong, J.; Cai, Y.; Wang, J.; Wen, J.; Peng, L.; Ji, H.; Jiang, S.; Guo, D. Chitosan modified squalene nanostructured lipid carriers as a promising adjuvant for freeze-dried ovalbumin vaccine. Int. J. Biol. Macromol. 2021, 188, 855–862. [Google Scholar] [CrossRef]
- Rodolfo, C.; Eusébio, D.; Ventura, C.; Nunes, R.; Florindo, H.F.; Costa, D.; Sousa, Â. Design of Experiments to Achieve an Efficient Chitosan-Based DNA Vaccine Delivery System. Pharmaceutics 2021, 13, 1369. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, B.; Shi, C.; Sun, Y.; Jin, Z.; Hu, G. Intranasal immunization with O-2′-Hydroxypropyl trimethyl ammonium chloride chitosan nanoparticles loaded with Newcastle disease virus DNA vaccine enhances mucosal immune response in chickens. J. Nanobiotechnol. 2021, 19, 240. [Google Scholar] [CrossRef]
- Lopes, P.D.; Okino, C.H.; Fernando, F.S.; Pavani, C.; Casagrande, V.M.; Lopez, R.F.V.; Montassier, M.F.S.; Montassier, H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine 2018, 36, 2630–2636. [Google Scholar] [CrossRef]
- Dhakal, S.; Renu, S.; Ghimire, S.; Shaan Lakshmanappa, Y.; Hogshead, B.T.; Feliciano-Ruiz, N.; Lu, F.; HogenEsch, H.; Krakowka, S.; Lee, C.W.; et al. Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front. Immunol. 2018, 9, 934. [Google Scholar] [CrossRef]
- Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.; Wu, C.W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M. Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization. Vaccines 2021, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Alu, A.; Yang, J.; He, C.; Hong, W.; Cheng, Z.; Yang, L.; Li, J.; Wang, Z.; Wang, W.; et al. Cationic nanocarriers as potent adjuvants for recombinant S-RBD vaccine of SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 291. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, D.; Taniai, M.; Ariyasu, H.; Taniguchi, M.; Aga, M.; Ariyasu, T.; Ohta, T.; Fukuda, S. Cholesteryl Pullulan Encapsulated TNF-α Nanoparticles Are an Effective Mucosal Vaccine Adjuvant against Influenza Virus. BioMed Res. Int. 2015, 2015, 471468. [Google Scholar] [CrossRef] [Green Version]
- Wuertz, K.M.; Barkei, E.K.; Chen, W.H.; Martinez, E.J.; Lakhal-Naouar, I.; Jagodzinski, L.L.; Paquin-Proulx, D.; Gromowski, G.D.; Swafford, I.; Ganesh, A.; et al. A SARS-CoV-2 spike ferritin nanoparticle vaccine protects hamsters against Alpha and Beta virus variant challenge. NPJ Vaccines 2021, 6, 129. [Google Scholar] [CrossRef]
- Bakkari, M.A.; Valiveti, C.K.; Kaushik, R.S.; Tummala, H. Toll-like Receptor-4 (TLR4) Agonist-Based Intranasal Nanovaccine Delivery System for Inducing Systemic and Mucosal Immunity. Mol. Pharm. 2021, 18, 2233–2241. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, W.; Guo, M.; Huang, M.; Gu, Y.; Wang, T.; Ni, G.; Ming, D. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem. Eng. 2021, 418, 129392. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Sessa, G.; Weissmann, G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968, 9, 310–318. [Google Scholar] [CrossRef]
- Allison, A.G.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef] [PubMed]
- Wasan, E.K.; Syeda, J.; Strom, S.; Cawthray, J.; Hancock, R.E.; Wasan, K.M.; Gerdts, V. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine 2019, 37, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of class B CpG ODN onto DOTAP/DC-chol liposome in nasal vaccine formulations augments antigen-specific immune responses in mice. BMC Res. Notes 2017, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Durrer, P.; Glück, U.; Spyr, C.; Lang, A.B.; Zurbriggen, R.; Herzog, C.; Glück, R. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine 2003, 21, 4328–4334. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hou, C.; Bai, Y.; Wang, R.; Liu, J. Protein Assembly: Versatile Approaches to Construct Highly Ordered Nanostructures. Chem. Rev. 2016, 116, 13571–13632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, S.; Liu, M.; Kang, W.; Xia, J. Self-Assembled Multienzyme Nanostructures on Synthetic Protein Scaffolds. ACS Nano 2019, 13, 11343–11352. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Citiulo, F.; Crosatti, C.; Cattivelli, L.; Biselli, C. Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines. Plants 2021, 10, 1828. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Cid, R.; Bolívar, J. Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Polla Ravi, S.; Shamiya, Y.; Chakraborty, A.; Elias, C.; Paul, A. Biomaterials, biological molecules, and polymers in developing vaccines. Trends Pharmacol. Sci. 2021, 42, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.L.; Horsted, E.W.; et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef]
- Lu, J.; Lu, G.; Tan, S.; Xia, J.; Xiong, H.; Yu, X.; Qi, Q.; Yu, X.; Li, L.; Yu, H.; et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020, 30, 936–939. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Mweetwa, L.L.; Ntemi, P.V.; Chikukwa, M.T.R.; Matafwali, S.K.; Mwila, C.; Mudenda, S.; Katandula, J.; Walker, R.B. Nano-Biomimetic Drug Delivery Vehicles: Potential Approaches for COVID-19 Treatment. Molecules 2020, 25, 5952. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Zuo, J.; Wen, M.; Lei, M.; Xiao, X.; Liu, Z. PLGA-Der p1 Vaccine Inhibited Tumor Growth in a Murine Model of Lung Cancer. Arch. Med. Res. 2015, S0188-4409, 00289-1. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., III; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Felipe, L.; Vercruysse, T.; Sharma, S.; Ma, J.; Lemmens, V.; Van Looveren, D.; Arkalagud Javarappa, M.P.; Boudewijns, R.; Malengier-Devlies, B.; Liesenborghs, L.; et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 2021, 590, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Patki, M.; Palekar, S.; Reznik, S.; Patel, K. Self-injectable extended release formulation of Remdesivir (SelfExRem): A potential formulation alternative for COVID-19 treatment. Int. J. Pharm. 2021, 597, 120329. [Google Scholar] [CrossRef]
- Tan, Q.; He, L.; Meng, X.; Wang, W.; Pan, H.; Yin, W.; Zhu, T.; Huang, X.; Shan, H. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J. Nanobiotechnol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef]
- Kingstad-Bakke, B.; Toy, R.; Lee, W.; Pradhan, P.; Vogel, G.; Marinaik, C.B.; Larsen, A.; Gates, D.; Luu, T.; Pandey, B.; et al. Polymeric Pathogen-Like Particles-Based Combination Adjuvants Elicit Potent Mucosal T Cell Immunity to Influenza a Virus. Front. Immunol. 2020, 11, 559382. [Google Scholar] [CrossRef]
- Ainai, A.; Ichinohe, T.; Tamura, S.; Kurata, T.; Sata, T.; Tashiro, M.; Hasegawa, H. Zymosan enhances the mucosal adjuvant activity of poly(I:C) in a nasal influenza vaccine. J. Med. Virol. 2010, 82, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Dengl, S.; Sustmann, C.; Brinkmann, U. Engineered hapten-binding antibody derivatives for modulation of pharmacokinetic properties of small molecules and targeted payload delivery. Immunol. Rev. 2016, 270, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffar, K.A.; Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. The Role of Size in Development of Mucosal Liposome-Lipopeptide Vaccine Candidates Against Group A Streptococcus. Med. Chem. 2016, 13, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Yan, M.; Yu, Q.; Yang, Q. Characteristics of nasal-associated lymphoid tissue (NALT) and nasal absorption capacity in chicken. PLoS ONE 2013, 8, e84097. [Google Scholar] [CrossRef]
- Nainu, F.; Abidin, R.S.; Bahar, M.A.; Frediansyah, A.; Emran, T.B.; Rabaan, A.A.; Dhama, K.; Harapan, H. SARS-CoV-2 reinfection and implications for vaccine development. Hum. Vaccines Immunother. 2020, 16, 3061–3073. [Google Scholar] [CrossRef]
- Cohen, J.I.; Burbelo, P.D. Reinfection with SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis. 2020, 73, e4223–e4228. [Google Scholar] [CrossRef]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, D.; et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet. Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Investig. 2010, 39, 303–355. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors for COVID-19 Vaccine Development. Viruses 2021, 13, 317. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; López-Estévez, A.M.; Cordeiro, A.S.; Dacoba, T.G.; Crecente-Campo, J.; Torres, D.; Alonso, M.J. Nanotechnologies for the delivery of biologicals: Historical perspective and current landscape. Adv. Drug Deliv. Rev. 2021, 176, 113899. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses 2021, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Hall, D.; Omoru, O.B.; Gill, H.M.; Smith, S.; Janga, S.C. Mutational Landscape and Interaction of SARS-CoV-2 with Host Cellular Components. Microorganisms 2021, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.M.; Chou, C.W.; Kuo, H.C.; Javanmardi, K.; Hsieh, C.L.; Goldsmith, J.; DiVenere, A.M.; Le, K.C.; Wrapp, D.; Byrne, P.O.; et al. Expression and characterization of SARS-CoV-2 spike proteins. Nat. Protoc. 2021, 16, 5339–5356. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
| Nasal Candidate Nanovaccines | NPs | Types of NPs | Developers | Functions |
|---|---|---|---|---|
| A DNA nanovaccine, modified vaccinia ankara expressing SARS-CoV-2 S and N antigens and based with quil-A-loaded chitosan (QAC) [63] | Quil-A-loaded chitosan (QAC) | Polysaccharide | Shaswath et al. | Protection of plasmid integrity and as a adjuvant |
| A SARS-CoV-2 spike ferritin nanoparticle vaccine (NCT04784767) [68] | Ferritin and Army Liposomal Formulation QS21 (SpFN-ALFQ) | Self-assembled proteins | Kathryn et al. | Enhanced cellular uptake of ferritin and lipidosome NPs, and protection of antigens by liposomes |
| A Toll-like receptor-4 (TLR4) agonist-based intranasal nanovaccine [69] | inulin acetate (InAc) | Polysaccharide | Kathryn et al. | As toll-like receptor-4 (TLR4) agonist |
| A inhalable nanovaccine with biomimetic coronavirus structure [70] | poly(I:C) and biomimetic pulmonary surfactant (bio-PS) liposomes | Nano-biomimetic delivery vehicles | Bin Zheng et al. | Completely simulate the structure of the coronavirus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ding, Y.; Yao, J.; Zhang, M.; Zhang, Y.; Xie, Z.; Zuo, J. Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19. Vaccines 2022, 10, 405. https://doi.org/10.3390/vaccines10030405
Huang J, Ding Y, Yao J, Zhang M, Zhang Y, Xie Z, Zuo J. Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19. Vaccines. 2022; 10(3):405. https://doi.org/10.3390/vaccines10030405
Chicago/Turabian StyleHuang, Jialu, Yubo Ding, Jingwei Yao, Minghui Zhang, Yu Zhang, Zhuoyi Xie, and Jianhong Zuo. 2022. "Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19" Vaccines 10, no. 3: 405. https://doi.org/10.3390/vaccines10030405
APA StyleHuang, J., Ding, Y., Yao, J., Zhang, M., Zhang, Y., Xie, Z., & Zuo, J. (2022). Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19. Vaccines, 10(3), 405. https://doi.org/10.3390/vaccines10030405





