Towards a Four-Component GMMA-Based Vaccine against Shigella
Abstract
:1. Introduction
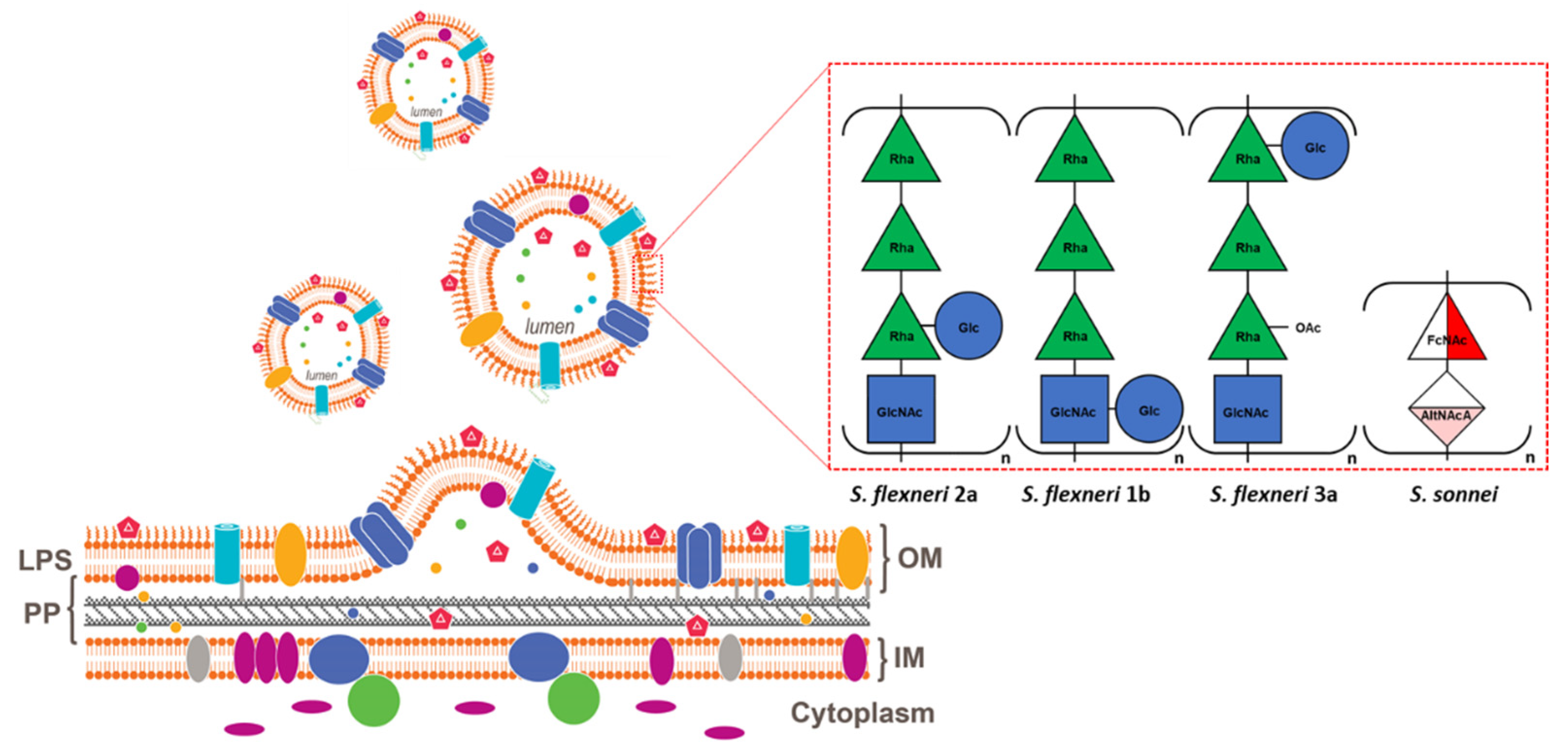
2. Generalized Modules for Membrane Antigens Platform
3. Shigella Generalized Modules for Membrane Antigens
3.1. Preclinical Experience
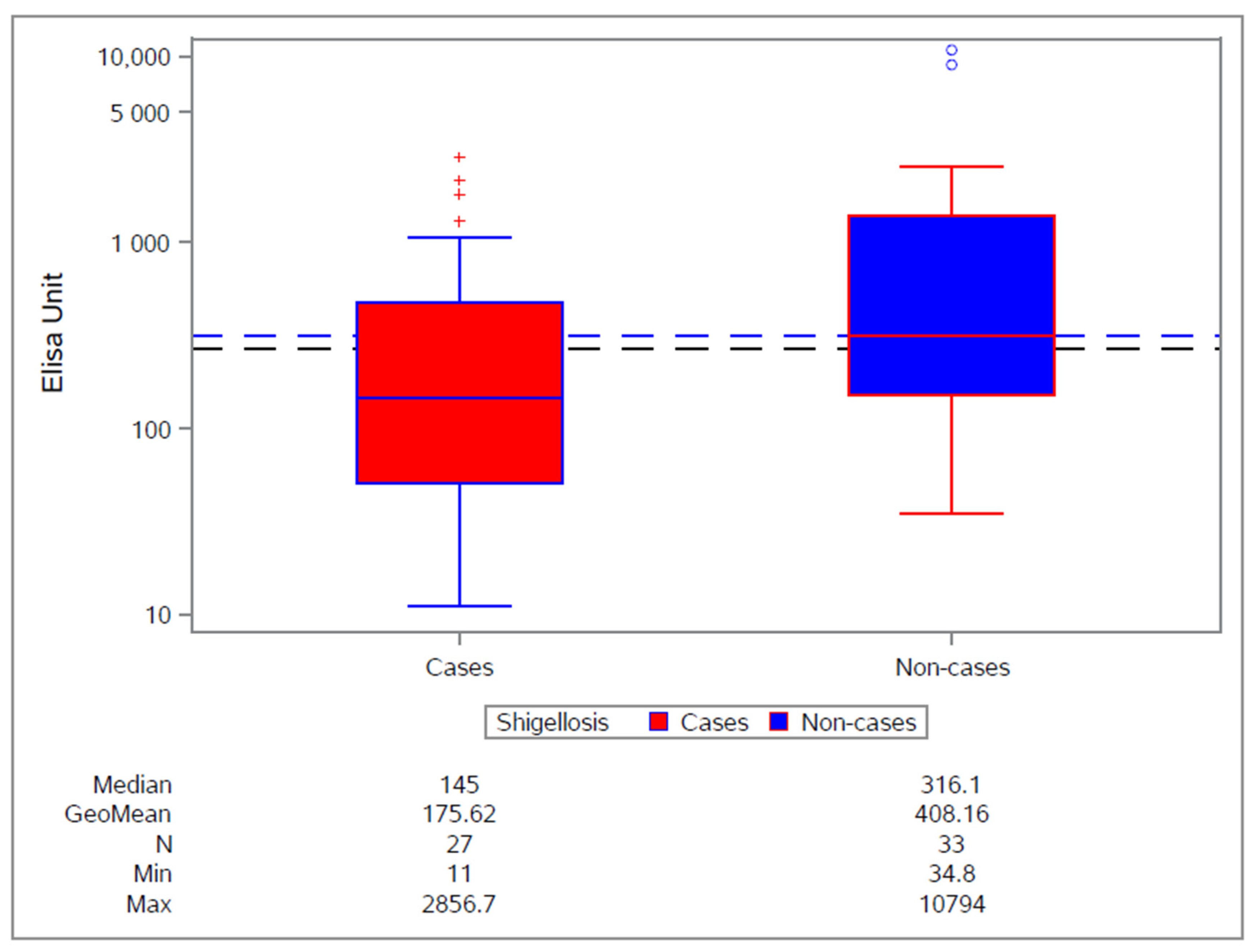
3.2. Clinical Experience with S. sonnei Monocomponent GMMA Vaccine
4. Moving toward a Four-Component Shigella GMMA-Based Vaccine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Tickell, K.D.; Sharmin, R.; Deichsel, E.L.; Lamberti, L.M.; Walson, J.L.; Faruque, A.S.G.; Pavlinac, P.B.; Kotloff, K.L.; Chisti, M.J. The effect of acute malnutrition on enteric pathogens, moderate-to-severe diarrhoea, and associated mortality in the Global Enteric Multicenter Study cohort: A post-hoc analysis. Lancet Glob. Health 2020, 8, e215–e224. [Google Scholar] [CrossRef] [Green Version]
- Klontz, K.C.; Singh, N. Treatment of drug-resistant Shigella infections. Expert Rev. Anti-Infect. Ther. 2015, 13, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 11 February 2022).
- Wu, Y.; Lau, H.K.; Lee, T.; Lau, D.K.; Payne, J. In Silico Serotyping Based on Whole-Genome Sequencing Improves the Accuracy of Shigella Identification. Appl. Environ. Microbiol. 2019, 85, e00165-19. [Google Scholar] [CrossRef] [Green Version]
- Formal, S.B.; Oaks, E.V.; Olsen, R.E.; Wingfield-Eggleston, M.; Snoy, P.J.; Cogan, J.P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 1991, 164, 533–537. [Google Scholar] [CrossRef]
- DuPont, H.L.; Hornick, R.B.; Snyder, M.J.; Libonati, J.P.; Formal, S.B.; Gangarosa, E.J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J. Infect. Dis. 1972, 125, 12–16. [Google Scholar] [CrossRef]
- Herrington, D.A.; Van de Verg, L.; Formal, S.B.; Hale, T.L.; Tall, B.D.; Cryz, S.J.; Tramont, E.C.; Levine, M.M. Studies in volunteers to evaluate candidate Shigella vaccines: Further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 1990, 8, 353–357. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Losonsky, G.A.; Wasserman, S.S.; Hale, T.L.; Taylor, D.N.; Sadoff, J.C.; Levine, M.M. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: Clinical experience and implications for Shigella infectivity. Vaccine 1995, 13, 1488–1494. [Google Scholar] [CrossRef]
- Ferreccio, C.; Prado, V.; Ojeda, A.; Cayyazo, M.; Abrego, P.; Guers, L.; Levine, M.M. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am. J. Epidemiol. 1991, 134, 614–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, D.; Green, M.S.; Block, C.; Slepon, R.; Ofek, I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J. Clin. Microbiol. 1991, 29, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.; Green, M.S.; Block, C.; Rouach, T.; Ofek, I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J. Infect. Dis. 1988, 157, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Mel, D.M.; Terzin, A.L.; Vuksić, L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull. World Health Organ. 1965, 32, 647–655. [Google Scholar]
- Mel, D.M.; Arsić, B.L.; Nikolić, B.D.; Radovanić, M.L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull. World Health Organ. 1968, 39, 375–380. [Google Scholar]
- Mel, D.; Gangarosa, E.J.; Radovanovic, M.L.; Arsic, B.L.; Litvinjenko, S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull. World Health Organ. 1971, 45, 457–464. [Google Scholar]
- Mel, D.M.; Arsic, B.L.; Radovanovic, M.L.; Litvinjenko, S.A. Live oral Shigella vaccine: Vaccination schedule and the effect of booster dose. Acta Microbiol. Acad. Sci. Hung. 1974, 21, 109–114. [Google Scholar]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, R.; Kaminski, R.W.; Porter, C.; Choy, R.K.M.; White, J.A.; Fleckenstein, J.M.; Cassels, F.; Bourgeois, L. Vaccines for Protecting Infants from Bacterial Causes of Diarrheal Disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Rogawski McQuade, E.T.; Liu, J.; Kang, G.; Kosek, M.N.; Lima, A.A.M.; Bessong, P.O.; Samie, A.; Haque, R.; Mduma, E.R.; Shrestha, S.; et al. Protection From Natural Immunity Against Enteric Infections and Etiology-Specific Diarrhea in a Longitudinal Birth Cohort. J. Infect. Dis. 2020, 222, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Hochberg, A.; Ashkenazi, S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccin. Immunother. 2019, 15, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.; Block, C.; Green, M.S.; Lowell, G.; Ofek, I. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J. Clin. Microbiol. 1989, 27, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Robin, G.; Cohen, D.; Orr, N.; Markus, I.; Slepon, R.; Ashkenazi, S.; Keisari, Y. Characterization and quantitative analysis of serum IgG class and subclass response to Shigella sonnei and Shigella flexneri 2a lipopolysaccharide following natural Shigella infection. J. Infect. Dis. 1997, 175, 1128–1133. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.; Micoli, F.; Necchi, F.; Pizza, M.; Berlanda Scorza, F.; Rossi, O. GMMA-Based Vaccines: The Known and The Unknown. Front. Immunol. 2021, 12, 715393. [Google Scholar] [CrossRef]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High yield production process for Shigella outer membrane particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [Green Version]
- Adu-Bobie, J.; Lupetti, P.; Brunelli, B.; Granoff, D.; Norais, N.; Ferrari, G.; Grandi, G.; Rappuoli, R.; Pizza, M. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 2004, 72, 1914–1919. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, G.; Garaguso, I.; Adu-Bobie, J.; Doro, F.; Taddei, A.R.; Biolchi, A.; Brunelli, B.; Giuliani, M.M.; Pizza, M.; Norais, N.; et al. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: Proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 2006, 6, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Bernadac, A.; Gavioli, M.; Lazzaroni, J.C.; Raina, S.; Lloubes, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [Google Scholar] [CrossRef] [Green Version]
- Berlanda Scorza, F.; Doro, F.; Rodríguez-Ortega, M.J.; Stella, M.; Liberatori, S.; Taddei, A.R.; Serino, L.; Gomes Moriel, D.; Nesta, B.; Fontana, M.R.; et al. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli DeltatolR IHE3034 mutant. Mol. Cell. Proteom. 2008, 7, 473–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Berlanda Scorza, F.; Norais, N.; Laera, D.; Giusti, F.; Pierleoni, A.; et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles 2013, 2, 20181. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 2016, 23, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [Green Version]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Maggiore, L.; Yu, L.; Omasits, U.; Rossi, O.; Dougan, G.; Thomson, N.R.; Saul, A.; Choudhary, J.S.; Gerke, C. Quantitative proteomic analysis of Shigella flexneri and Shigella sonnei Generalized Modules for Membrane Antigens (GMMA) reveals highly pure preparations. Int. J. Med. Microbiol. 2016, 306, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.; Gasperini, G.; Rossi, O.; Aruta, M.G.; Raso, M.M.; Alfini, R.; Biagini, M.; Necchi, F.; Micoli, F. Dissecting the contribution of O-Antigen and proteins to the immunogenicity of Shigella sonnei generalized modules for membrane antigens (GMMA). Sci. Rep. 2021, 11, 906. [Google Scholar] [CrossRef]
- Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F.; et al. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines 2020, 8, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasperini, G.; Raso, M.M.; Arato, V.; Aruta, M.G.; Cescutti, P.; Necchi, F.; Micoli, F. Effect of O-Antigen Chain Length Regulation on the Immunogenicity of Shigella and Salmonella Generalized Modules for Membrane Antigens (GMMA). Int. J. Mol. Sci. 2021, 22, 1309. [Google Scholar] [CrossRef] [PubMed]
- Arato, V.; Oldrini, D.; Massai, L.; Gasperini, G.; Necchi, F.; Micoli, F. Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice. Microorganisms 2021, 9, 2360. [Google Scholar] [CrossRef]
- Meloni, E.; Colucci, A.M.; Micoli, F.; Sollai, L.; Gavini, M.; Saul, A.; Di Cioccio, V.; MacLennan, C.A. Simplified low-cost production of O-antigen from Salmonella Typhimurium Generalized Modules for Membrane Antigens (GMMA). J. Biotechnol. 2015, 198, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef]
- Schager, A.E.; Dominguez-Medina, C.C.; Necchi, F.; Micoli, F.; Goh, Y.S.; Goodall, M.; Flores-Langarica, A.; Bobat, S.; Cook, C.N.L.; Arcuri, M.; et al. IgG Responses to Porins and Lipopolysaccharide within an Outer Membrane-Based Vaccine against Nontyphoidal Salmonella Develop at Discordant Rates. MBio 2018, 9, e02379-17. [Google Scholar] [CrossRef] [Green Version]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 41, 10428–10433. [Google Scholar] [CrossRef] [Green Version]
- Koeberling, O.; Ispasanie, E.; Hauser, J.; Rossi, O.; Pluschke, G.; Caugant, D.A.; Saul, A.; MacLennan, C.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [Google Scholar] [CrossRef] [Green Version]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, e1800376. [Google Scholar] [CrossRef] [Green Version]
- Micoli, F.; Alfini, R.; Giannelli, C. Methods for Assessment of OMV/GMMA Quality and Stability. Methods Mol. Biol. 2022, 2414, 227–279. [Google Scholar] [CrossRef]
- De Benedetto, G.; Cescutti, P.; Giannelli, C.; Rizzo, R.; Micoli, F. Multiple Techniques for Size Determination of Generalized Modules for Membrane Antigens from Salmonella typhimurium and Salmonella enteritidis. ACS Omega 2017, 2, 8282–8289. [Google Scholar] [CrossRef]
- Palmieri, E.; Arato, V.; Oldrini, D.; Ricchetti, B.; Aruta, M.G.; Pansegrau, W.; Marchi, S.; Giusti, F.; Ferlenghi, I.; Rossi, O.; et al. Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency. Vaccines 2021, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Rosenqvist, E.; Høiby, E.A.; Bjune, G.; Aase, A.; Halstensen, A.; Lehmann, A.K.; Paulssen, J.; Holst, J.; Michaelsen, T.E.; Nøkleby, H.; et al. Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine. Dev. Biol. Stand. 1998, 92, 323–333. [Google Scholar] [PubMed]
- Citiulo, F.; Necchi, F.; Mancini, F.; Rossi, O.; Aruta, M.G.; Gasperini, G.; Alfini, R.; Rondini, S.; Micoli, F.; Rappuoli, R.; et al. Rationalizing the design of a broad coverage Shigella vaccine based on evaluation of immunological cross-reactivity among S. flexneri serotypes. PLoS Negl. Trop. Dis. 2021, 15, e0009826. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Conti, V.; Ferruzzi, P.; Ndiaye, A.G.W.; Parker, S.; McNeal, M.M.; Dickey, M.; Granada, J.P.; Cilio, G.L.; De Ryck, I.; et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: Results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine 2021, 39, 101076. [Google Scholar] [CrossRef] [PubMed]
- Ndungo, E.; Pasetti, M.F. Functional antibodies as immunological endpoints to evaluate protective immunity against Shigella. Hum. Vaccin. Immunother. 2020, 16, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef]
- Whitfield, C.; Williams, D.M.; Kelly, S.D. Lipopolysaccharide O-antigens-bacterial glycans made to measure. J. Biol. Chem. 2020, 295, 10593–10609. [Google Scholar] [CrossRef]
- Caboni, M.; Pédron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Scire, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muturi-Kioi, V.; Lewis, D.; Launay, O.; Leroux-Roels, G.; Anemona, A.; Loulergue, P.; Bodinham, C.L.; Aerssens, A.; Groth, N.; Saul, A.; et al. Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review. PLoS ONE 2016, 11, e0157385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micoli, F.; Rossi, O.; Conti, V.; Launay, O.; Sciré, A.S.; Aruta, M.G.; Nakakana, U.N.; Marchetti, E.; Rappuoli, R.; Saul, A.; et al. Antibodies Elicited by the Shigella sonnei GMMA Vaccine in Adults Trigger Complement-Mediated Serum Bactericidal Activity: Results From a Phase 1 Dose Escalation Trial Followed by a Booster Extension. Front. Immunol. 2021, 12, 671325. [Google Scholar] [CrossRef]
- Fries, L.F.; Montemarano, A.D.; Mallett, C.P.; Taylor, D.N.; Hale, T.L.; Lowell, G.H. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect. Immun. 2001, 69, 4545–4553. [Google Scholar] [CrossRef] [Green Version]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Scire, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schutte, L.D.; Auerbach, J.; et al. Booster Vaccination With GVGH Shigella sonnei 1790GAHB GMMA Vaccine Compared to Single Vaccination in Unvaccinated Healthy European Adults: Results From a Phase 1 Clinical Trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Obiero, C.W.; Ndiaye, A.G.W.; Scire, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schutte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef] [Green Version]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558. [Google Scholar] [CrossRef]
- WHO. Annual Report WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Di Benedetto, R.; Alfini, R.; Carducci, M.; Aruta, M.G.; Lanzilao, L.; Acquaviva, A.; Palmieri, E.; Giannelli, C.; Necchi, F.; Saul, A.; et al. Novel Simple Conjugation Chemistries for Decoration of GMMA with Heterologous Antigens. Int. J. Mol. Sci. 2021, 22, 10180. [Google Scholar] [CrossRef] [PubMed]
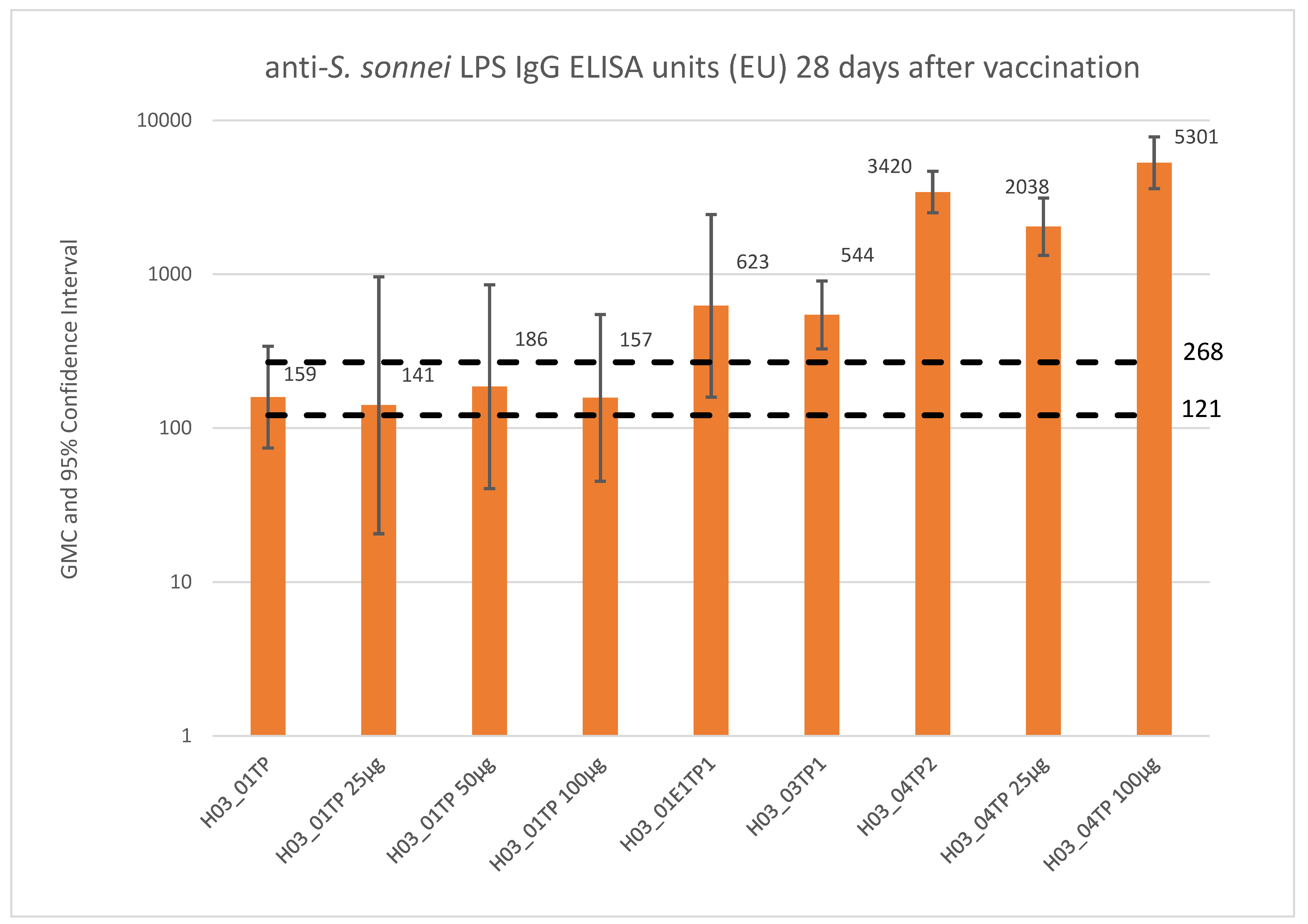
| Study Phase; (Country) No. of Doses; Type of Study | Study Population (Age) | Test Product(s)—Dose (Route) | No. of Subjects Exposed | Study Status/ Report Status |
|---|---|---|---|---|
| H03_01TP; (France) 3 doses; Phase 1 | Adults (18 to 45 years) | 1790GAHB—0.06/1 µg (IM) 1790GAHB—0.3/5 µg (IM) 1790GAHB—1.5/25 µg (IM) 1790GAHB—3/50 µg (IM) 1790GAHB—6/100 µg (IM) Placebo (IM) | 8 9 8 8 9 8 | Study completed/CSR available in final form |
| H03_02TP; (UK) 3 doses; Phase 1 | Adults (18 to 45 years) | 1790GAHB—0.006/0.1 µg (ID) 1790GAHB—0.06/1 µg (ID) 1790GAHB—0.6/10 µg (ID) 1790GAHB—0.3/5 µg (IN) 1790GAHB—1.2/20 µg (IN) 1790GAHB—4.8/80 µg (IN) 1790GAHB—0.3/5 µg (IM) Placebo (ID) Placebo (IN) Placebo (IM) | 4 6 6 4 6 6 6 6 7 1 | Study completed/CSR available in final form |
| H03_04TP; (Kenya) 2 doses; Phase 2a | Adults (18 to 45 years) | 1790GAHB—1.5/25 µg (IM) 1790GAHB—6/100 µg (IM) Menveo followed by Boostrix (IM) | 22 26 24 | Study completed/CSR available in final form |
| H03_01E1TP; (France) 1 dose; Phase 1 | Adults (22 to 50 years) | 1790GAHB—1.5/25 µg (IM) | 35 | Study completed/CSR available in final form |
| H03_03TP (US) 2 doses, Phase 2b | Adults (18 to 50 years) | 1790GAHB—1.5/25 µg (IM) Placebo (IM) | 36 | Study completed/CSR available in final form. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. https://doi.org/10.3390/vaccines10020328
Micoli F, Nakakana UN, Berlanda Scorza F. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines. 2022; 10(2):328. https://doi.org/10.3390/vaccines10020328
Chicago/Turabian StyleMicoli, Francesca, Usman N. Nakakana, and Francesco Berlanda Scorza. 2022. "Towards a Four-Component GMMA-Based Vaccine against Shigella" Vaccines 10, no. 2: 328. https://doi.org/10.3390/vaccines10020328
APA StyleMicoli, F., Nakakana, U. N., & Berlanda Scorza, F. (2022). Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines, 10(2), 328. https://doi.org/10.3390/vaccines10020328







